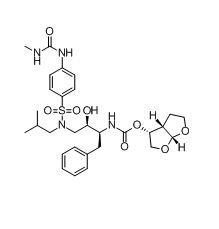
Darunavir N-methyl urea impurity
| Product Name | Darunavir N-methyl urea impurity |
|---|---|
| Alternate Names | Darunavir Impurities, Impurities of Darunavir |
| CAT No. | CS-O-34249 |
| CAS No. | 1451010-37-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 604.71 g/mol |
| Mol. For. | C₂₉H₄₀N₄O₈S |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Darunavir |
| Smileys | O=C(N[C@H]([C@H](O)CN(CC(C)C)[S](=O)(C1=CC=C(NC(NC)=O)C=C1)=O)CC2=CC=CC=C2)O[C@@H]3[C@@](CCO4)([H])[C@@]4([H])OC3 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Darunavir is a medication used to treat HIV infection. It works by inhibiting the protease enzyme, which is responsible for cleaving the viral polyproteins into their functional proteins, thus preventing the formation of new viral particles. However, during the manufacturing process of darunavir, impurities may be formed, and one of these impurities is the N-methyl urea impurity.
The N-methyl urea impurity is a byproduct formed during the synthesis of darunavir. It is a small molecule with a molecular weight of 102.12g/mol. The chemical formula of N-methyl urea is CH5N2O, and it is a white crystalline solid at room temperature. This impurity is not clinically relevant and does not affect the efficacy or safety of the drug. However, it is important to monitor and control the levels of this impurity during the production of darunavir to ensure product quality.
The N-methyl urea impurity can be quantified using analytical methods such as high-performance liquid chromatography (HPLC) or gas chromatography (GC). These methods involve separating the impurity from other components in the sample and measuring its concentration. The maximum allowed level of N-methyl urea impurity in darunavir is 0.1% according to the guidelines set by regulatory agencies.
In conclusion, the N-methyl urea impurity is a minor byproduct formed during the synthesis of darunavir. Although it is not clinically relevant, it is important to monitor and control its levels during the production process to ensure product quality.
Get an Instant Quote
Related Compounds
Darunavir Impurity 11 | Darunavir Impurity 46 | Darunavir Impurity 7 (S,S-Isomer) | Darunavir Diamino impurity | Darunavir Impurity 10 | Darunavir Impurity 16 | Darunavir Amine dimer impurity | Darunavir Impurity 8 (R,R-Isomer) | Darunavir СВZ furan impurity | Darunavir Impurity D | Darunavir Impurity A Enantiomer | Darunavir СВZ urea impurity | N-[3S-benzyloxycarbonylamino-2R-hydroxy-4-phenyl]-N-isobutylamine | Darunavir Urea impurity | Darunavir Impurity 38 | Darunavir СВZ amino impurity | Darunavir Carbamic Acid Methyl Ester | Darunavir Isomer 2 | Darunavir Nitro complex impurity | Darunavir impurity B | Darunavir Difuranyl impurity | Darunavir Impurity 23 | Darunavir Isomer 1 | Darunavir furan dimer impurity |