Etofenamate - Impurity G
| Product Name | Etofenamate - Impurity G |
|---|---|
| Alternate Names | Etofenamate Impurities, Impurities of Etofenamate |
| CAT No. | CS-O-37467 |
| CAS No. | 32508-98-8 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 325.28 g/mol |
| Mol. For. | C₁₆H₁₄F₃NO₃ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Etofenamate |
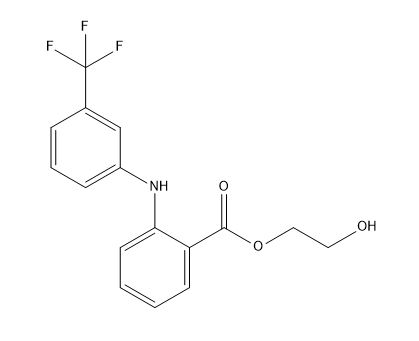
| Smileys | O=C(OCCO)C1=CC=CC=C1NC2=CC=CC(C(F)(F)F)=C2 |
| Canonical Smiles | C1=CC=C(C(=C1)C(=O)OCCO)NC2=CC=CC(=C2)C(F)(F)F |
| InchIKey | QTAIVYIVAODSBA-UHFFFAOYSA-N |
| Inchl | InChI=1S/C16H14F3NO3/c17-16(18,19)11-4-3-5-12(10-11)20-14-7-2-1-6-13(14)15(22)23-9-8-21/h1-7,10,20-21H,8-9H2 |
| IUPAC | 2-hydroxyethyl 2-[3-(trifluoromethyl)anilino]benzoate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Etofenamate is a nonsteroidal anti-inflammatory drug (NSAID) used to relieve pain and inflammation in conditions such as rheumatoid arthritis, osteoarthritis, and sports injuries. It works by inhibiting the production of prostaglandins, which are chemicals that cause inflammation and pain in the body.
Etofenamate is available in various forms, such as tablets, capsules, and topical creams. It is usually applied topically to the affected area, where it is absorbed through the skin and into the underlying tissues. The recommended dosage and duration of treatment depend on the severity of the condition and the individual's response to the medication.
Impurity G is a byproduct that is formed during the synthesis of etofenamate. It is a minor impurity that is usually present in small amounts in etofenamate formulations. The chemical structure of impurity G is closely related to that of etofenamate, but it has a slightly different composition that affects its pharmacological properties.
Studies have shown that impurity G has a lower potency and slower onset of action compared to etofenamate. It also has a longer half-life and is eliminated from the body more slowly. However, impurity G is generally considered to be safe and well-tolerated, and its presence in etofenamate formulations does not significantly affect the overall efficacy or safety of the medication.
Get an Instant Quote
Related Compounds
N-nitroso Etofenamate | Etofenamate - Impurity D | Etofenamate EP Impurity E |