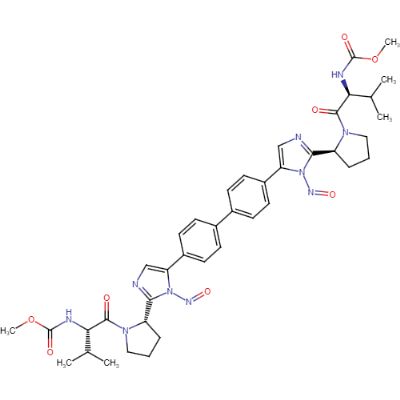
Daclatasvir dihydrochloride nitrosamine impurity
| Product Name | Daclatasvir dihydrochloride nitrosamine impurity |
|---|---|
| Alternate Names | Daclatasvir Impurities, Impurities of Daclatasvir |
| CAT No. | CS-O-43412 |
| CAS No. | Not Available |
| Category | Impurities |
| Stock | Enquire |
| Mol. Wt. | 796.87 g/mol |
| Mol. For. | C40H48N10O8 |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Daclatasvir |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Daclatasvir dihydrochloride is a medication used to treat chronic hepatitis C virus (HCV) infection. It works by inhibiting the HCV nonstructural protein 5A (NS5A), which is necessary for viral replication. However, during the manufacturing process of Daclatasvir dihydrochloride, a nitrosamine impurity can form. Nitrosamines are a class of compounds that have been shown to be carcinogenic in animal studies. The nitrosamine impurity in Daclatasvir dihydrochloride has been identified as N-nitrosodimethylamine (NDMA).
NDMA is a highly toxic and carcinogenic compound that has been classified as a probable human carcinogen by the International Agency for Research on Cancer (IARC). Exposure to NDMA can cause damage to the liver, kidneys, and other organs. Therefore, it is essential to monitor and control the levels of NDMA in Daclatasvir dihydrochloride to ensure that the medication is safe for use.
To avoid exposure to NDMA, it is important to follow the recommended dosage and administration of Daclatasvir dihydrochloride and to only use the medication as prescribed by a healthcare professional. Patients should also be aware of any potential side effects and report them to their healthcare provider immediately. It is also recommended that patients undergo regular liver function tests while taking Daclatasvir dihydrochloride to monitor for any signs of liver damage.
Get an Instant Quote
Related Compounds
Daclatasvir Impurity-X | Daclatasvir Tert butyl ester | Daclatasvir Impurity-H | Daclatasvir RRSS Isomer | Daclatasvir Impurity-E | Daclatasvir Impurity-G | N-Acetyl Daclatasvir | Daclatasvir Impurity-P | Monodes(N-carboxymethyl)valine Daclatasvir | Daclatasvir Impurity-B | Daclatasvir Impurity-Z | Daclatasvir Impurity-Y | Daclatasvir SRSR Isomer | Daclatasvir RRRR Isomer Enantiomer (2HCl) | Daclatasvir Dihydrochloride | Daclatasvir Impurity 4 | Daclatasvir Impurity-R | Daclatasvir Impurity-A | Daclatasvir Impurity-Q | Daclatasvir Enantiomer 2HCl | Daclatasvir Impurity 5 (SRSS-Isomer) | Daclatasvir Impurity-D | Daclatasvir Impurity-C | Daclatasvir Impurity-T |