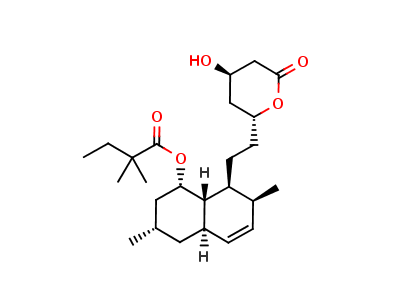
Simvastatin Impurity K
| Product Name | Simvastatin Impurity K |
|---|---|
| Alternate Names | Simvastatin Impurities, Impurities of Simvastatin |
| CAT No. | CS-P-00262 |
| CAS No. | Not Available |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 420.58 g/mol |
| Mol. For. | C₂₅H₄₀O₅ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Simvastatin |
| Purity | 95% |
| Therapeutic | Anti-Diabetic |
| Smileys | CCC(C)(C)C(O[C@H]1C[C@@H](C)C[C@]2([H])C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(O3)=O)[C@@]12[H])=O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Simvastatin Impurity K, also known as 2,2-dimethyl-6-oxo-1,3-dioxane-4-acetic acid, is a chemical compound that is commonly used as a reference standard in the analysis of Simvastatin. Simvastatin Impurity K is a degradation product of Simvastatin and its presence in the drug substance and drug product is monitored to ensure the quality of Simvastatin.
Simvastatin is a cholesterol-lowering medication that is used to reduce the risk of heart attack, stroke, and other cardiovascular diseases. It works by inhibiting the enzyme HMG-CoA reductase, which is responsible for the production of cholesterol in the liver. Simvastatin Impurity K is a byproduct of the breakdown of Simvastatin in the body and is excreted in the urine.
In chemical terms, Simvastatin Impurity K is a dioxane derivative and belongs to the class of organic compounds known as lactones. It has a molecular weight of 182.17 g/mol and a melting point of 143-146°C. Simvastatin Impurity K is a white to off-white powder that is soluble in water and organic solvents such as methanol and acetonitrile.
In conclusion, Simvastatin Impurity K is an important reference standard used in the analysis of Simvastatin. Its chemical properties and usage play a crucial role in ensuring the quality and efficacy of Simvastatin as a cholesterol-lowering medication.
Get an Instant Quote
Related Compounds
Simvastatin EP Impurity D | Simvastatin EP Impurity C | Simvastatin Acid Glycerol Ester | Simvastatin EP Impurity A | Simvastatin Acid Methyl Ester | Simvastatin, 1-Pyreneacetyl Ester | Simvastatin 4'-Methyl Ether | Simvastatin pivaloyl Dehydro impurity | Simvastatin EP Impurity G | Simvastatin (3S)-Hydroxy Impurity | Simvastatin 6-Oxo Isomer | Simvastatin EP Impurity E | Simvastatin (6R)-Hydroxy Isomer | Simvastatin EP Impurity B | Simvastatin (3R)-Hydroxy Impurity | Simvastatin EP Impurity K | Simvastatin pivaloyl impurity | Simvastatin Anhydro Acid Sodium Salt | Simvastatin (6S)-Hydroxy Isomer | Simvastatin Acid Ethyl Ester |