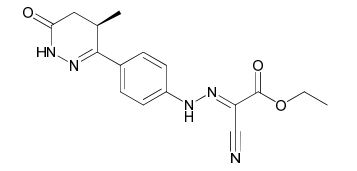
Levosimendan Cyanoacetate Hydrazone Impurity
| Product Name | Levosimendan Cyanoacetate Hydrazone Impurity |
|---|---|
| Alternate Names | Levosimendan Impurities, Impurities of Levosimendan |
| CAT No. | CS-P-01117 |
| CAS No. | Not Available |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 327.34 g/mol |
| Mol. For. | C₁₆H₁₇N₅O₃ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Levosimendan |
| Smileys | C[C@H](C1)C(C2=CC=C(NN=C(C#N)C(OCC)=O)C=C2)=NNC1=O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Levosimendan Cyanoacetate Hydrazone Impurity is a chemical impurity that is often found in pharmaceutical preparations containing the active ingredient levosimendan. Levosimendan is a calcium sensitizer and potassium channel opener used in the treatment of heart failure.
Levosimendan Cyanoacetate Hydrazone Impurity is a byproduct of the synthesis of levosimendan and is not intentionally added to pharmaceutical preparations. However, its presence can affect the efficacy and safety of the medication. Therefore, it is important to monitor and control the level of this impurity in pharmaceutical products.
Chemically, Levosimendan Cyanoacetate Hydrazone Impurity is a hydrazone derivative of cyanoacetic acid. It is a colorless to pale yellow solid with a melting point of 103-105°C. It is soluble in organic solvents such as methanol, ethanol, and acetonitrile but insoluble in water.
The usage of Levosimendan Cyanoacetate Hydrazone Impurity is limited to its detection and quantification in pharmaceutical preparations containing levosimendan. Analytical methods such as high-performance liquid chromatography (HPLC) and gas chromatography (GC) are commonly used to detect and quantify this impurity. The acceptable limit for Levosimendan Cyanoacetate Hydrazone Impurity in pharmaceutical preparations is 0.1% or less.
Get an Instant Quote
Related Compounds
Levosimendan Impurity | Levosimendan Related compound E | Levosimendan Related compound F | Levosimendan Impurity 16 | Levosimendan Impurity 3 | Levosimendan Cyanoacetamide Hydrazone Impurity | Levosimendan Impurity 18 | Levosimendan Impurity 6 | Levosimendan Dimer |