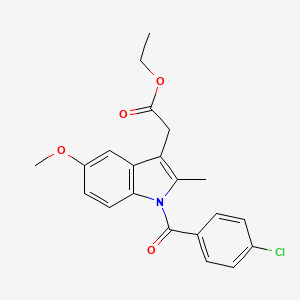
Indomethacin ethyl ester
| Product Name | Indomethacin ethyl ester |
|---|---|
| Alternate Names | Indomethacin Impurities, Impurities of Indomethacin |
| CAT No. | CS-P-08096 |
| CAS No. | 16401-99-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | Not Available |
| Mol. For. | Not Available |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Indomethacin |
| Canonical Smiles | CCOC(=O)CC1=C(N(C2=C1C=C(C=C2)OC)C(=O)C3=CC=C(C=C3)Cl)C |
| InchIKey | COIRSVPTDJIIKY-UHFFFAOYSA-N |
| Inchl | InChI=1S/C21H20ClNO4/c1-4-27-20(24)12-17-13(2)23(19-10-9-16(26-3)11-18(17)19)21(25)14-5-7-15(22)8-6-14/h5-11H,4,12H2,1-3H3 |
| IUPAC | ethyl 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Indomethacin ethyl ester is a derivative of the anti-inflammatory drug indomethacin. It is commonly used in the treatment of arthritis, gout, and other inflammatory conditions. The ethyl ester form of indomethacin is more lipophilic than the parent compound, which allows for better absorption and distribution throughout the body. Indomethacin ethyl ester is also less irritating to the gastrointestinal tract than indomethacin, making it a more tolerable option for patients.
The chemical structure of indomethacin ethyl ester is similar to that of indomethacin, with the addition of an ethyl group to the carboxylic acid functional group. This modification increases the molecule's stability and enhances its solubility in organic solvents. The ester bond can be hydrolyzed by esterases in the body, releasing the active indomethacin molecule.
Indomethacin ethyl ester works by inhibiting the production of prostaglandins, which are responsible for causing inflammation and pain. It achieves this by blocking the activity of the enzyme cyclooxygenase (COX). COX is responsible for converting arachidonic acid into prostaglandins, so by inhibiting this enzyme, indomethacin ethyl ester reduces the amount of prostaglandins produced.
In conclusion, indomethacin ethyl ester is a useful drug in the treatment of inflammatory conditions. Its chemical properties allow for better absorption and distribution throughout the body, and its mechanism of action involves the inhibition of prostaglandin production through blocking COX activity.
Get an Instant Quote
Related Compounds
Indomethacin Impurity D | O-Desmethyl-N-deschlorobenzoyl Indomethacin | Indomethacin EP Impurity J | Indomethacin Impurity 11 | Indomethacin Analogue | Indomethacin Impurity 9 | Indomethacin Diamide | Indomethacin Methyl Ester | Indomethacin Impurity 10 | Indomethacin PEG ester | Indomethacin-α-monoglyceride | Indomethacin Isopropyl Ester | Indomethacin Impurity 12 | O-Desmethyl Indomethacin | Indomethacin Impurity E | Indomethacin impurity F | Indomethacin Impurity 15 | Indomethacin impurity F | N-(4-Acetamidophenyl)indomethacin Amide | Indomethacin Impurity G |