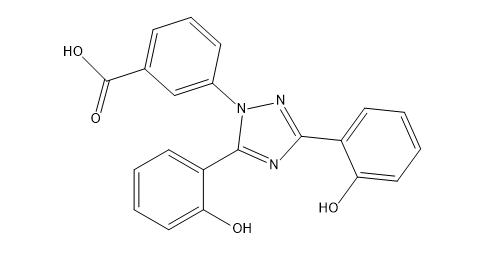
Deferasirox Impurity D
| Product Name | Deferasirox Impurity D |
|---|---|
| Alternate Names | Deferasirox Impurities, Impurities of Deferasirox |
| CAT No. | CS-P-08260 |
| CAS No. | 2254105-61-6 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 373.36 g/mol |
| Mol. For. | C₂₁H₁₅N₃O₄ |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Deferasirox |
| Purity | 95% |
| Smileys | O=C(O)C1=CC=CC(N2N=C(C3=CC=CC=C3O)N=C2C4=CC=CC=C4O)=C1 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Deferasirox Impurity D, also known as 4-[(5Z)-5-[(E)-2-(2,4-dimethylphenyl)hydrazin-1-ylidene]-2-oxo-1,3-thiazolidin-3-yl]benzoic acid, is a synthetic organic compound that is commonly used as an analytical reference standard in research and development laboratories. This impurity is a byproduct of the manufacturing process of Deferasirox, which is an iron-chelating agent used in the treatment of chronic iron overload conditions such as thalassemia and other blood disorders.
Chemically, Deferasirox Impurity D is a thiazolidinone derivative with a benzene ring substituted with a hydrazine group and a carboxylic acid group. It is a yellow to orange crystalline powder that is soluble in organic solvents such as methanol and ethanol but is insoluble in water.
In terms of usage, Deferasirox Impurity D is primarily used as a reference standard for the identification and quantification of impurities in Deferasirox drug substance and drug product. It is also used as a starting material for the synthesis of other Deferasirox impurities that may be present in the drug substance. The purity of Deferasirox Impurity D is critical to ensure accurate results in analytical testing, and it is typically analyzed using high-performance liquid chromatography (HPLC) or gas chromatography-mass spectrometry (GC-MS).
Overall, Deferasirox Impurity D is an important analytical tool for the quality control and development of Deferasirox drug products, and its chemical properties and usage make it a valuable reference standard in the pharmaceutical industry.
Get an Instant Quote
Related Compounds
Deferasirox Impurity 22 | Deferasirox Diacyl Impurity | Deferasirox Impurity F | Deferasirox bis salicylamide impurity | Deferasirox Ethyl Ester | Deferasirox Isopropyl Ester | Deferasirox Amide Impurity | Deferasirox Impurity 3 | Deferasirox Salicyloyl Ester | Deferasirox Hydrazino Impurity | Deferasirox Impurity 25 | Deferasirox Impurity 4 | Deferasirox Impurity 3 | Deferasirox Phase 1 impurity | Azobenzene-4,4'-dicarboxylic Acid | Deferasirox Impurity 9 |