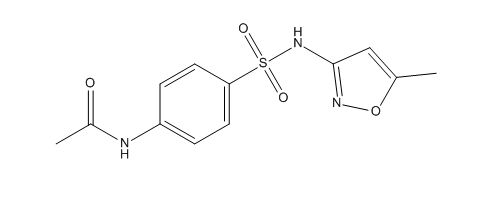
Sulfamethoxazole EP Impurity A
| Product Name | Sulfamethoxazole EP Impurity A |
|---|---|
| CAT No. | CS-T-01135 |
| CAS No. | 21312-10-7 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 295.31 g/mol |
| Mol. For. | C₁₂H₁₃N₃O₄S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Purity | 95% |
| Smileys | CC1=CC(=NO1)NS(=O)(=O)C2=CC=C(C=C2)NC(=O)C |
| Canonical Smiles | CC1=CC(=NO1)NS(=O)(=O)C2=CC=C(C=C2)NC(=O)C |
| InchIKey | GXPIUNZCALHVBA-UHFFFAOYSA-N |
| Inchl | InChI=1S/C12H13N3O4S/c1-8-7-12(14-19-8)15-20(17,18)11-5-3-10(4-6-11)13-9(2)16/h3-7H,1-2H3,(H,13,16)(H,14,15) |
| IUPAC | N-[4-[(5-methyl-1,2-oxazol-3-yl)sulfamoyl]phenyl]acetamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Sulfamethoxazole EP Impurity A is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard for the identification and quantification of impurities in Sulfamethoxazole (SMX) drug products. Sulfamethoxazole is a widely used antibiotic that belongs to the family of sulfonamides, which are synthetic antimicrobial agents that inhibit the growth of bacteria. Sulfamethoxazole EP Impurity A is a known impurity that can be present in SMX drug products, and its identification and quantification are necessary to ensure the safety and efficacy of the final drug product.
The chemical formula of Sulfamethoxazole EP Impurity A is C12H14N4O3S, and its molecular weight is 298.33 g/mol. The compound has a melting point of 220-225°C and is soluble in water, ethanol, and dimethyl sulfoxide. Sulfamethoxazole EP Impurity A is a yellowish-brown powder that is stable under normal conditions. It is a derivative of SMX and is formed during the manufacturing process of SMX drug products.
The usage of Sulfamethoxazole EP Impurity A is mainly for analytical purposes, such as quality control testing and method development. It is used as a reference standard to identify and quantify impurities in SMX drug products, which ensures the safety and efficacy of the final drug product. The impurity is also used in research and development to understand the formation and degradation pathways of SMX drug products.
Get an Instant Quote
This page contains information about Sulfamethoxazole EP Impurity A. You can buy Sulfamethoxazole EP Impurity A from Clearsynth at best competitive price with assured price guarantee. Clearsynth offers best quality Sulfamethoxazole EP Impurity A