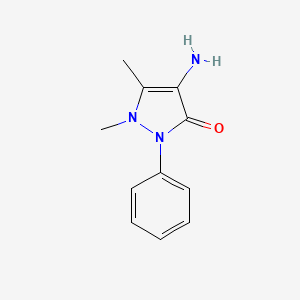
Metamizole EP Impurity B
| Product Name | Metamizole EP Impurity B |
|---|---|
| Alternate Names | Metamizole Impurities, Impurities of Metamizole |
| CAT No. | CS-T-03236 |
| CAS No. | 83-07-8 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 203.24 g/mol |
| Mol. For. | C₁₁H₁₃N₃O |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Metamizole |
| Purity | Not less than 90 % |
| Smileys | CC1=C(C(=O)N(N1C)C2=CC=CC=C2)N |
| Canonical Smiles | CC1=C(C(=O)N(N1C)C2=CC=CC=C2)N |
| InchIKey | RLFWWDJHLFCNIJ-UHFFFAOYSA-N |
| Inchl | InChI=1S/C11H13N3O/c1-8-10(12)11(15)14(13(8)2)9-6-4-3-5-7-9/h3-7H,12H2,1-2H3 |
| IUPAC | 4-amino-1,5-dimethyl-2-phenylpyrazol-3-one |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Metamizole EP Impurity B is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard for analytical purposes. It is a known impurity of the active pharmaceutical ingredient (API) Metamizole, also known as Dipyrone, which is used as a pain reliever and fever reducer.
Metamizole EP Impurity B is a synthetic compound that belongs to the class of pyrazolones. It has a molecular formula of C13H12N2O2 and a molecular weight of 228.25 g/mol. This impurity is typically produced during the synthesis of Metamizole and can be found in small quantities in the final product.
In terms of usage, Metamizole EP Impurity B is primarily used as a reference standard in analytical testing methods to identify and quantify the impurity in Metamizole API. It is also utilized in research and development studies to understand the properties and behavior of the compound.
While Metamizole EP Impurity B is not intended for therapeutic use, it is important to ensure that its presence in Metamizole API is within acceptable limits to ensure the safety and efficacy of the final product. Analytical methods utilizing this reference standard are critical in maintaining the quality of pharmaceutical products containing Metamizole as the active ingredient.
Get an Instant Quote
Related Compounds
N-Nitroso Metamizole EP Impurity C | Metamizole Impurity A | Metamizole Impurity C Sulfate salt | Metamizole Impurity 4 | Metamizole Impurity 3 | Metamizole Impurity 2 | Metamizole EP Impurity C HCl | Metamizole Impurity 5 | Metamizole Impurity C |