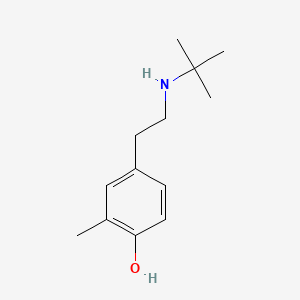
Salbutamol EP Impurity H
| Product Name | Salbutamol EP Impurity H |
|---|---|
| Alternate Names | Salbutamol Impurities, Impurities of Salbutamol |
| CAT No. | CS-T-09400 |
| CAS No. | 132183-64-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 207.3 g/mol |
| Mol. For. | C13H21NO |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Salbutamol |
| Purity | Not less than 95 % |
| Smileys | OC1=CC=C(CCNC(C)(C)C)C=C1C |
| Canonical Smiles | CC1=C(C=CC(=C1)CCNC(C)(C)C)O |
| InchIKey | NZVRVWRGZWEPRX-UHFFFAOYSA-N |
| Inchl | InChI=1S/C13H21NO/c1-10-9-11(5-6-12(10)15)7-8-14-13(2,3)4/h5-6,9,14-15H,7-8H2,1-4H3 |
| IUPAC | 4-[2-(tert-butylamino)ethyl]-2-methylphenol |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Salbutamol EP Impurity H is a chemical compound that is commonly used in the pharmaceutical industry. It is also known as 4-(2-hydroxyethyl)-1-methylpiperidine-4-carboxylic acid and is considered to be an impurity in Salbutamol, which is a bronchodilator medication used to treat asthma and other respiratory conditions.
The usage of Salbutamol EP Impurity H is mainly in the development and testing of Salbutamol products. It is used as a reference standard in the quality control of Salbutamol formulations. The impurity is also used in research to study the metabolism and pharmacology of Salbutamol.
Chemically, Salbutamol EP Impurity H is a piperidine derivative with a molecular weight of 173.22 g/mol. It is a white crystalline powder that is soluble in water and alcohol. The compound is stable under normal storage conditions and has a purity of ≥98%.
In terms of safety, Salbutamol EP Impurity H is classified as a non-hazardous substance. However, it is recommended that it be handled with care and in accordance with good laboratory practices.
In conclusion, Salbutamol EP Impurity H is an important chemical compound in the pharmaceutical industry. Its usage as a reference standard in the quality control of Salbutamol formulations and in research makes it a valuable tool for the development of new and effective treatments for respiratory conditions.
Get an Instant Quote
Related Compounds
Salbutamol EP Impurity L Hydrochloride | Colterol | Levalbuterol Related Compound E Hydrochloride | Salbutamol EP Impurity B | Salbutamol Impurity N Acetate Salt | Salbutamol Acetonide | Salbutamol EP Impurity I | Salbutamol Related Compound 1 | Salbutamol Impurity L | N-Benzyl Salbutamol Hydrochloride | Salbutamol Acetonide Methyl Ether | Salbutamol Impurity P | Salbutamol EP Impurity E | Salbutamol EP Impurity K (hemihydrate) | Salbutamol EP Impurity D Sulfate salt | Salbutamol Glyoxal Impurity | Salbutamol EP Impurity D | Salbutamol Impurity K | Salbutamol EP Impurity C | Salbutamol diethyl ether | Salbutamol EP Impurity G | Salbutamol Impurity O | Salbutamol EP Impurity A |