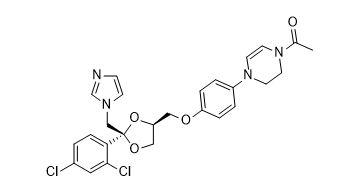
Ketoconazole Impurity A
| Product Name | Ketoconazole Impurity A |
|---|---|
| CAT No. | CS-T-14910 |
| CAS No. | 254912-63-5 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 529.4 g/mol |
| Mol. For. | C26H26Cl2N4O4 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Purity | Not less than 90 % |
| Therapeutic | Anti-Fungals |
| Smileys | ClC1=C(C=CC(Cl)=C1)[C@@]2(O[C@@H](COC3=CC=C(N4CCN(C(C)=O)C=C4)C=C3)CO2)CN5C=CN=C5 |
| Canonical Smiles | CC(=O)N1CCN(C=C1)C2=CC=C(C=C2)OCC3COC(O3)(CN4C=CN=C4)C5=C(C=C(C=C5)Cl)Cl |
| InchIKey | GHVFISLXKPKRQU-OZXSUGGESA-N |
| Inchl | InChI=1S/C26H26Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-10,12,14,18,23H,11,13,15-17H2,1H3/t23-,26-/m0/s1 |
| IUPAC | 1-[4-[4-[[(2R,4S)-2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-2,3-dihydropyrazin-1-yl]ethanone |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ketoconazole Impurity A is a chemical compound that is commonly used in the manufacturing of ketoconazole, which is an antifungal medication. This impurity is a highly pure and stable form of ketoconazole that is used as a reference standard in quality control testing of ketoconazole formulations. It is also used as a chemical marker to identify the presence of impurities in the drug substance.
Ketoconazole Impurity A is a white to off-white crystalline powder that has a molecular formula of C21H20Cl2N2O3. It has a melting point of 188-190°C and a purity of not less than 98%. This impurity is soluble in methanol and slightly soluble in water. Its chemical structure is similar to that of ketoconazole, and it is considered a minor impurity in the manufacturing of ketoconazole.
The usage of Ketoconazole Impurity A is critical in ensuring the quality and safety of ketoconazole-based medications. It is used in the quality control testing of ketoconazole formulations to ensure that the drug is free of any impurities that could be harmful to patients. This impurity is also used in research studies to understand the chemical properties and behavior of ketoconazole.
In summary, Ketoconazole Impurity A is a minor impurity that is critical in ensuring the safety and quality of ketoconazole-based medications. Its chemical properties and behavior are essential in understanding the behavior of ketoconazole, making it a valuable tool in research studies.
Get an Instant Quote
This page contains information about Ketoconazole Impurity A. You can buy Ketoconazole Impurity A from Clearsynth at best competitive price with assured price guarantee. Clearsynth offers best quality Ketoconazole Impurity A