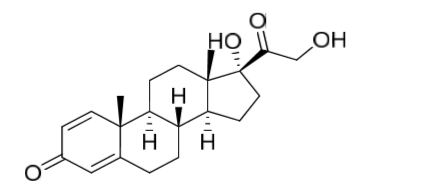
Prednisolone EP Impurity J
| Product Name | Prednisolone EP Impurity J |
|---|---|
| Alternate Names | Prednisolone Impurities, Impurities of Prednisolone |
| CAT No. | CS-T-15501 |
| CAS No. | 1807-14-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 344.4 g/mol |
| Mol. For. | C21H28O4 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Prednisolone |
| Therapeutic | Anti-Asthma / COPD |
| Canonical Smiles | CC12CCC3C(C1CCC2(C(=O)CO)O)CCC4=CC(=O)C=CC34C |
| InchIKey | BVAYTJBBDODANA-OBQKJFGGSA-N |
| Inchl | InChI=1S/C21H28O4/c1-19-8-5-14(23)11-13(19)3-4-15-16(19)6-9-20(2)17(15)7-10-21(20,25)18(24)12-22/h5,8,11,15-17,22,25H,3-4,6-7,9-10,12H2,1-2H3/t15-,16+,17+,19+,20+,21+/m1/s1 |
| IUPAC | (8R,9S,10R,13S,14S,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Prednisolone EP Impurity J is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard for the analysis of prednisolone, which is a synthetic glucocorticoid used to treat a variety of inflammatory conditions. The compound is also known as 20-dihydrocortisol and is a metabolite of cortisol, the primary stress hormone in humans. Prednisolone EP Impurity J is a white to off-white crystalline powder that is soluble in water, ethanol, and methanol.
The chemical formula of Prednisolone EP Impurity J is C21H32O5, and its molecular weight is 372.48 g/mol. The compound is classified as a steroid and is often used as a reference standard for the analysis of prednisolone in various pharmaceutical formulations. It is important to note that Prednisolone EP Impurity J is not intended for human consumption and should only be used for laboratory purposes.
In conclusion, Prednisolone EP Impurity J is a valuable reference standard in the pharmaceutical industry, used for the analysis of prednisolone in various formulations. The compound is a metabolite of cortisol and is classified as a steroid. While it is not intended for human consumption, it plays an important role in quality control and assurance in the production of pharmaceuticals.
Get an Instant Quote
Related Compounds
Prednisolone-21-Dimethyl amine | Prednisolone 21-Propionate | Prednisolone 20-ethyl Ester | Deoxyprednisolone-16-ene | Prednisolone Impurity 10 | Prednisolone Sodium Phosphate USP Impurity A | Prednisolone EP Impurity F | Prednisolone Impurity 9 | Prednisolone 11,21-Diacetate | Prednisolone sodium phosphate USP impurity F | Prednisolone D-Homo A derivative | Prednisolone metasulfobenzoate | Prednisolone 17-(Ethyl Carbonate) 21-Acetate | Prednisolone Caproate | Prednisolone-21-carboxylic Acid | Prednisolone EP Impurity A Hydrochloride | Prednisolone Impurity 16 | Prednisolone 21-Ethylcarbonate | D-Homo-16-alphahydroxy prednisolone | Prednisolone Dicarbonate | Methyl Prednisolone Impurity D (Isomer mixture-1 and 2) | Prednisolone Sodium Phosphate USP Impurity E | Methyl Prednisolone sulfate triethyl amine salt | Prednisolone-17-acetate | Prednisolone 21-formate | Prednisolone D-homo B Derivative | Prednisolone sodium phosphate Impurity Isomer 1 | Prednisolone Hemisuccinate | Prednisolone Sodium Diphosphate Derivative | Prednisolone Sodium Phosphate 11-Phospahte | Prednisolone sodium phosphate Isomer III | Methyl Prednisolone sulfate | Prednisolone Tebutate | Prednisolone Dehydrated (Ether form) | Prednisolone Impurity 2 |