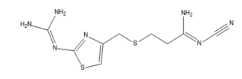
Famotidine EP impurity G
| Product Name | Famotidine EP impurity G |
|---|---|
| Alternate Names | Famotidine Impurities, Impurities of Famotidine |
| CAT No. | CS-T-15632 |
| CAS No. | 76823-97-7 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 283.38 g/mol |
| Mol. For. | C₉H₁₃N₇S₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Famotidine |
| Purity | 95% |
| Therapeutic | Anti ulcer |
| Smileys | C1=C(N=C(S1)N=C(N)N)CSCCC(=NC#N)N |
| Canonical Smiles | C1=C(N=C(S1)N=C(N)N)CSCCC(=NC#N)N |
| InchIKey | DFHYHFAHYAJKDU-UHFFFAOYSA-N |
| Inchl | InChI=1S/C9H13N7S2/c10-5-14-7(11)1-2-17-3-6-4-18-9(15-6)16-8(12)13/h4H,1-3H2,(H2,11,14)(H4,12,13,15,16) |
| IUPAC | N'-cyano-3-[[2-(diaminomethylideneamino)-1,3-thiazol-4-yl]methylsulfanyl]propanimidamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Famotidine EP impurity G is a chemical compound that is used in pharmaceutical manufacturing and research. It is a known impurity that is often present in small quantities in batches of Famotidine, a medication that is commonly used to treat gastrointestinal conditions such as acid reflux and stomach ulcers.
Chemically, Famotidine EP impurity G is also known as N''-[(5-amino-1,3,4-thiadiazol-2-yl)methyl]-N-[(methylsulfonyl)amino] formamidine. It is a derivative of Famotidine and is produced during the manufacturing process. Its chemical structure is similar to that of Famotidine, with the addition of a thiadiazole ring and a methylsulfonyl group.
While Famotidine EP impurity G is not a drug itself, it is important to monitor its presence in batches of Famotidine as it may affect the purity and potency of the medication. The acceptable limit for Famotidine EP impurity G in pharmaceuticals is typically set at a maximum of 0.1%.
Overall, Famotidine EP impurity G is an important compound that is monitored in pharmaceutical manufacturing to ensure the safety and efficacy of Famotidine as a medication.
Get an Instant Quote
Related Compounds
Famotidine Impurity 1 Hydrochloride | FAMOTIDINE IMPURITY C | Famotidine Impurity I | N-sulfamoylacrylimidamide | Famotidine Acid Methyl Ester | Famotidine Impurity 10 HCl | N-Nitroso Famotidine -II | Famotidine EP Impurity B (Dimaleate salt) | Famotidine Impurity H | Famotidine EP impurity B | Famotidine Impurity 8 | N-Nitroso Famotidine - I | Famotidine EP impurity A hydrochloride | Famotidine EP impurity D | Famotidine Adduct Impurity | N-Nitroso famotidine-III | Famotidine EP impurity E | Famotidine EP Impurity I Hydrochloride | Famotidine Nitroso Impurity 1 | Dediaminosulfonyl Hydroxymethyl Famotidine | Famotidine glucose adduct | Famotidine Sulfamide dimer | Famotidine Related Compound A Dihydrochloride | Famotidine EP Impurity F |