Ranitidine EP impurity A
| Product Name | Ranitidine EP impurity A |
|---|---|
| Alternate Names | Ranitidine Impurities, Impurities of Ranitidine |
| CAT No. | CS-T-19636 |
| CAS No. | 72126-78-4 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 497.67 g/mol |
| Mol. For. | C₂₂H₃₅N₅O₄S₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ranitidine |
| Therapeutic | Anti ulcer |
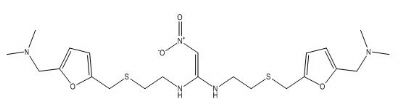
| Smileys | CN(C)CC1=CC=C(O1)CSCCNC(=C[N+](=O)[O-])NCCSCC2=CC=C(O2)CN(C)C |
| Canonical Smiles | CN(C)CC1=CC=C(O1)CSCCNC(=C[N+](=O)[O-])NCCSCC2=CC=C(O2)CN(C)C |
| InchIKey | VTVSRHZKBPARSF-UHFFFAOYSA-N |
| Inchl | InChI=1S/C22H35N5O4S2/c1-25(2)13-18-5-7-20(30-18)16-32-11-9-23-22(15-27(28)29)24-10-12-33-17-21-8-6-19(31-21)14-26(3)4/h5-8,15,23-24H,9-14,16-17H2,1-4H3 |
| IUPAC | 1-N,1-N'-bis[2-[[5-[(dimethylamino)methyl]furan-2-yl]methylsulfanyl]ethyl]-2-nitroethene-1,1-diamine |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ranitidine is a popular medication used to treat various gastrointestinal disorders, including acid reflux and ulcers. Ranitidine EP impurity A, also known as N-nitrosodimethylamine (NDMA), is a chemical impurity that has been found in some batches of ranitidine products.
NDMA is classified as a probable human carcinogen, meaning that it has the potential to cause cancer in humans. It is believed to form as a result of the breakdown of ranitidine in the body or during the manufacturing process.
The presence of NDMA in ranitidine products has led to widespread concern and recalls of the medication. While the risk of cancer from short-term use of ranitidine containing NDMA is considered to be low, long-term exposure may pose a greater risk.
It is important for healthcare professionals and patients to be aware of the potential risks associated with NDMA in ranitidine products. Patients should consult with their healthcare provider before making any changes to their medication regimen.
Manufacturers of ranitidine products are working to ensure the safety of their products by implementing rigorous testing and quality control measures to detect and remove NDMA. Patients who have concerns about the safety of their medication should speak with their healthcare provider.
Get an Instant Quote
Related Compounds
Ranitidine N-Nitroso Impurity | Desmethyl Ranitidine | Ranitidine Related Compound A | Ranitidine Impurity F.HCl | Ranitidine N,S-Dioxide | Ranitidine EP Impurity B | Ranitidine EP Impurity J | Ranitidine Impurity 2 | Ranitidine oxalic acid | Ranitidine EP Impurity G | Ranitidine EP Impurity H | Ranitidine EP Impurity E | Ranitidine EP Impurity I | Ranitidine EP Impurity K | Ranitidine EP Impurity F | Ranitidine EP Impurity D |