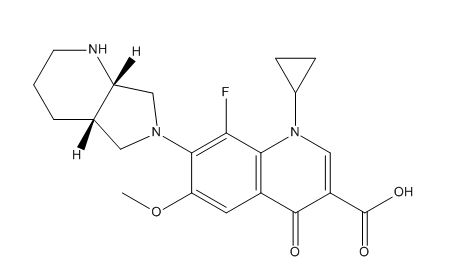
Moxifloxacin EP Impurity D
| Product Name | Moxifloxacin EP Impurity D |
|---|---|
| Alternate Names | Moxifloxacin Impurities, Impurities of Moxifloxacin |
| CAT No. | CS-T-24106 |
| CAS No. | 1029364-77-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 401.4 g/mol |
| Mol. For. | C21H24FN3O4 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Moxifloxacin |
| Therapeutic | Antibiotics |
| Smileys | COC1=C(C(=C2C(=C1)C(=O)C(=CN2C3CC3)C(=O)O)F)N4CC5CCCNC5C4 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Moxifloxacin EP Impurity D is a chemical compound that is commonly used as a reference standard or a quality control agent in the pharmaceutical industry. It is a synthetic compound that is closely related to the antibiotic drug Moxifloxacin, which is used to treat various bacterial infections. Moxifloxacin EP Impurity D is also known by its chemical name, 1-cyclopropyl-6-fluoro-8-methoxy-7-(4-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid.
The chemical structure and properties of Moxifloxacin EP Impurity D make it an ideal reference standard for the analysis and detection of Moxifloxacin in pharmaceutical formulations. It is often used in combination with other reference standards to ensure the accuracy and consistency of analytical results.
In terms of its chemical properties, Moxifloxacin EP Impurity D is a yellow crystalline powder that has a molecular weight of 413.42 g/mol. It is slightly soluble in water and highly soluble in organic solvents like methanol and acetonitrile. It has a melting point of 255-260°C and a purity of not less than 98%.
Overall, Moxifloxacin EP Impurity D is a valuable tool for the pharmaceutical industry, providing a reliable reference standard for the analysis and detection of Moxifloxacin in drug formulations. Its chemical properties and analytical characteristics make it an essential component in the quality control process for Moxifloxacin-containing drugs.
Get an Instant Quote
Related Compounds
Moxifloxacin Impurity 69 | ent-Moxifloxacin Hydrochloride | N-Allyloxycarbonyl Moxifloxacin | Moxifloxacin N-methyl Impurity | N-Nitroso-Moxifloxacin | Moxifloxacin Nitroso impurity 2 ( R,R Isomer) | N-Nitroso Moxifloxacin Related compound-A | Moxifloxacin EP Impurity D HCl salt | Moxifloxacin Difluoro Hydroxy Impurity | Moxifloxacin Related Compound B | N-Nitroso Moxifloxacin Related compound-B | N-Methyl Moxifloxacin Hydrochloride | Moxifloxacin Nitroso Impurity 2 | Moxifloxacin Nitroso impurity 1 ( R,R Isomer) | Moxifloxacin Nitroso R,R Isomer impurity | Moxifloxacin EP impurity B | Moxifloxacin Nitroso impurity 3 ( R,R Isomer) | Moxifloxacin Impurity 1 | Moxifloxacin Related Compound G HCl salt | Moxifloxacin EP Impurity A | Moxifloxacin Related Compound H | Moxifloxacin Methyl Ester | N-formyl moxifloxacin | N-Nitroso Moxifloxacin Related compound-C | Mono-Nitroso-Pyrrolopiperidine | Moxifloxacin Hydrochloride Monohydrate | Di-Nitroso- Pyrrolopiperidine | Moxifloxacin Impurity 2 | Moxifloxacin EP impurity E | Moxifloxacin Impurity D Hydrochloride (8-Fluoro-6-Methoxy Moxifloxacin) | Moxifloxacin Nitroso impurity 4 | N-Nitroso Desmethyl Moxifloxacin methyl ester | Moxifloxacin Impurity 38 | N-Nitroso Moxifloxacin Related compound-D | Moxifloxacin Related Compound F | N-Nitroso Moxifloxacin methyl ester |