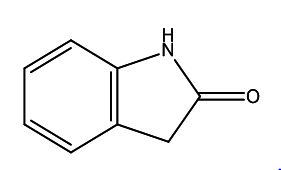
Diclofenac EP Impurity E
| Product Name | Diclofenac EP Impurity E |
|---|---|
| Alternate Names | Diclofenac Impurities, Impurities of Diclofenac |
| CAT No. | CS-T-29316 |
| CAS No. | 59-48-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 133.15 g/mol |
| Mol. For. | C₈H₇NO |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Diclofenac |
| Purity | 95% |
| Therapeutic | Anti-Migraines |
| Smileys | O=C1CC(C=CC=C2)=C2N1 |
| Canonical Smiles | C1C2=CC=CC=C2NC1=O |
| InchIKey | JYGFTBXVXVMTGB-UHFFFAOYSA-N |
| Inchl | InChI=1S/C8H7NO/c10-8-5-6-3-1-2-4-7(6)9-8/h1-4H,5H2,(H,9,10) |
| IUPAC | 1,3-dihydroindol-2-one |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Diclofenac EP Impurity E is a chemical compound that is commonly used in the pharmaceutical industry for quality control purposes. It is a known impurity in the production of Diclofenac, which is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammation.
Chemically, Diclofenac EP Impurity E is known as 2-Amino-3-methylbenzoic acid. Its molecular formula is C8H9NO2 and its molecular weight is 151.16 g/mol. It is a white to off-white powder with a melting point of approximately 174-176 °C.
In terms of usage, Diclofenac EP Impurity E is typically used as a reference standard in the analysis of Diclofenac samples. It is used to ensure the purity and quality of Diclofenac products and to identify any impurities or contaminants that may be present in the drug.
It is important to note that Diclofenac EP Impurity E is not intended for human consumption and should only be used in laboratory settings by trained professionals. It is also important to handle this compound with care as it may be harmful if ingested or inhaled.
Overall, Diclofenac EP Impurity E plays a crucial role in the production and quality control of Diclofenac products, ensuring their safety and efficacy for consumers.
Get an Instant Quote
Related Compounds
Diclofenac EP Impurity B | N-Nitroso-Diclofenac | Diclofenac Carboxylic Acid | Diclofenac Impurity C | Isopropyl ester of Diclofenac | Diclofenac Impurity D | Keto Diclofenac Sodium Salt | diclofenac sodium impurity IP (2-[(2, 6-dichlorophenyl) amino] phenyl]acetic acid) | diclofenac impurity F | Diclofenac glyoxillic acid | Diclofenac Impurity 2 | Diclofenac EP Impurity D | Diclofenac 2,5-Quinone Imine | Diclofenac Related Compound 3 | Diclofenac Dimer Impurity | Diclofenac tetradecyl ester | Diclofenac dodecyl ester |