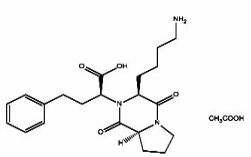
Lisinopril EP impurity C Acetate salt
| Product Name | Lisinopril EP impurity C Acetate salt |
|---|---|
| Alternate Names | Lisinopril Impurities, Impurities of Lisinopril |
| CAT No. | CS-T-31176 |
| CAS No. | 328385-86-0 (free base) |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 447.52 g/mol |
| Mol. For. | C₂₃H₃₃N₃O₆ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Lisinopril |
| Therapeutic | Anti-Hypertensives |
| Smileys | O=C(O)[C@@H](N([C@H]1CCCCN)C([C@@](CCC2)([H])N2C1=O)=O)CCC3=CC=CC=C3.CC(O)=O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Lisinopril EP impurity C is a commonly used pharmaceutical compound that belongs to the class of angiotensin-converting enzyme (ACE) inhibitors. It is used for the treatment of high blood pressure, heart failure, and to improve survival after a heart attack. Despite its effectiveness, the compound may contain impurities that affect its potency and safety. Lisinopril EP impurity C is one such impurity that can be found in small amounts in Lisinopril formulations.
Chemically, Lisinopril EP impurity C is known as (2S)-1-[2-[(1-carboxy-3-phenylpropyl)amino]-1-oxoethyl]-L-proline. It is a by-product of the synthesis of Lisinopril and can be formed during the manufacturing process or storage of the drug. The presence of impurity C in Lisinopril formulations can affect its efficacy and safety, hence it is important to monitor its levels and keep them within acceptable limits.
To ensure that Lisinopril EP impurity C does not cause harm to patients, regulatory bodies have set limits on its maximum allowed concentration in Lisinopril formulations. Pharmaceutical companies must adhere to these limits and ensure that their products meet the required quality standards. Analytical methods such as high-performance liquid chromatography (HPLC) are used to detect and quantify the impurity C levels in Lisinopril formulations.
In conclusion, Lisinopril EP impurity C is an important impurity that needs to be monitored in Lisinopril formulations to ensure their safety and efficacy. It is a by-product of the synthesis of Lisinopril and can be formed during the manufacturing process or storage of the drug. Regulatory bodies have set limits on its maximum allowed concentration, and analytical methods such as HPLC are used to detect and quantify its levels.
Get an Instant Quote
Related Compounds
Lisinopril EP Impurity G | Lisinopril EP Impurity I Acetate salt | N-(1-Carboxy-3-phenylpropyl)-S-lisinopril (Mixture of diastereomers) | Lisinopril EP Impurity J | Lisinopril Des-Proline dimer - II | Lisinopril EP Impurity A | Lisinopril EP Impurity F | Lisinopril-D8 | Lisinopril-D4 | Lisinopril EP Impurity D | N2-(1-Ethoxycarbonyl-3-oxo-3-phenylpropyl)-N6-trifluoroacetyl-L-lysine | Lisinopril SRS-Diastereomer | Lisinopril EP Impurity A | Lisinopril EP Impurity E | N-Benzyloxycarbonyl (S)-Lisinopril Ethyl Methyl Diester | Lisinopril Intermediate | N-trifluoroacetyl Lisinopril Intermediate | N-Benzyloxycarbonyl (S)-Lisinopril | N-Benzyloxycarbonyl Lisinopril Cyclohexyl Analogue Ethyl Methyl Diester |