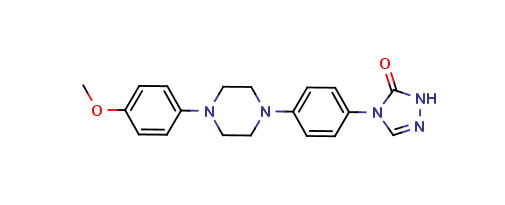
Itraconazole Methoxy Triazolone Impurity
| Product Name | Itraconazole Methoxy Triazolone Impurity |
|---|---|
| CAT No. | CS-T-32814 |
| CAS No. | 74853-07-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | Not Available |
| Mol. For. | C19H21N5O2 |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Therapeutic | Anti-Fungals |
| Smileys | O=C(NN=C1)N1C2=CC=C(N3CCN(C4=CC=C(OC)C=C4)CC3)C=C2 |
| Canonical Smiles | COC1=CC=C(C=C1)N2CCN(CC2)C3=CC=C(C=C3)N4C=NNC4=O |
| InchIKey | SEHQVBVJJRRRSG-UHFFFAOYSA-N |
| Inchl | InChI=1S/C19H21N5O2/c1-26-18-8-6-16(7-9-18)23-12-10-22(11-13-23)15-2-4-17(5-3-15)24-14-20-21-19(24)25/h2-9,14H,10-13H2,1H3,(H,21,25) |
| IUPAC | 4-[4-[4-(4-methoxyphenyl)piperazin-1-yl]phenyl]-1H-1,2,4-triazol-5-one |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Itraconazole Methoxy Triazolone Impurity is a chemical impurity that is commonly found in Itraconazole, an antifungal drug used to treat various fungal infections. This impurity is a byproduct of the synthesis process of Itraconazole and is present in small amounts in the final drug product.
The usage of Itraconazole Methoxy Triazolone Impurity is strictly limited to analytical purposes as it has no therapeutic value. It is mainly used as a reference standard for the quality control and analytical testing of Itraconazole drug products. The presence of this impurity in Itraconazole drug products is closely monitored and regulated by health agencies to ensure the safety and efficacy of the drug.
Chemically, Itraconazole Methoxy Triazolone Impurity is a triazole derivative that contains a methoxy group in its chemical structure. It is a white to off-white solid that is sparingly soluble in water and soluble in organic solvents. It is stable under normal storage conditions and does not undergo any significant degradation or reaction.
In conclusion, Itraconazole Methoxy Triazolone Impurity is an important reference standard for the quality control and analytical testing of Itraconazole drug products. Its usage is restricted to analytical purposes, and it has no therapeutic value. Its chemical properties make it a stable and reliable reference standard for analytical testing.
Get an Instant Quote
This page contains information about Itraconazole Methoxy Triazolone Impurity. You can buy Itraconazole Methoxy Triazolone Impurity from Clearsynth at best competitive price with assured price guarantee. Clearsynth offers best quality Itraconazole Methoxy Triazolone Impurity