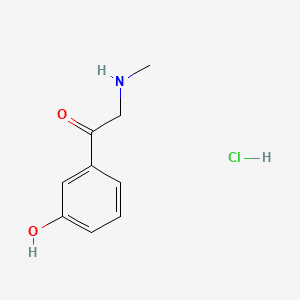
Phenylephrine EP Impurity C HCl
| Product Name | Phenylephrine EP Impurity C HCl |
|---|---|
| Alternate Names | Phenylephrine Impurities, Impurities of Phenylephrine |
| CAT No. | CS-T-39480 |
| CAS No. | 94240-17-2 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 201.65 g/mol |
| Mol. For. | C₉H₁₂ClNO₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Phenylephrine |
| Purity | 95% |
| Smileys | CNCC(=O)C1=CC(=CC=C1)O.Cl |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Phenylephrine EP Impurity C is an organic compound that is commonly used as a pharmaceutical reference standard in the development and manufacturing of pharmaceutical products. It is a secondary impurity of phenylephrine, a medication used primarily as a decongestant and to treat hypotension.
Phenylephrine EP Impurity C is a white, crystalline powder that is soluble in water and ethanol. It is classified as a secondary impurity, meaning that it is not present in significant quantities in the final pharmaceutical product but is used as a reference material to ensure the purity and quality of the active ingredient.
The chemical structure of Phenylephrine EP Impurity C is similar to that of phenylephrine, with the addition of a hydroxyl group on the benzene ring. This small difference in structure can have a significant impact on the activity and safety of the final pharmaceutical product.
The usage of Phenylephrine EP Impurity C is critical in the development and manufacturing of pharmaceutical products, as it ensures the purity and quality of the active ingredient. By using a reference standard, pharmaceutical manufacturers can accurately measure the purity and concentration of phenylephrine in their products, ensuring that they are safe and effective for patient use.
In conclusion, Phenylephrine EP Impurity C is an important reference standard in the pharmaceutical industry, used to ensure the purity and quality of phenylephrine and to maintain patient safety.
Get an Instant Quote
Related Compounds
Phenylephrine Impurity 33 | D-Phenylephrine sulfonate | Phenylephrine Impurity-41 | Phenylephrine Impurity-37 | O-Acetyl-(R)-phenylephrine Hydrochloride | Phenylephrine Citrate Adduct -2 | Phenylephrine impurity 43 | Phenylephrine EP Impurity A | Phenylephrine D-(+)-glucose Adduct | N-(2-Succinyl) Phenylephrine | L-Phenylephrine sulfonate | Phenylephrine Impurity D | Phenylephrine Impurity 4 | N-Nitroso Phenylephrine (Mixture of Isomers) | rac Benzyl Phenylephrine | Phenylephrine Impurity 9 | Phenylephrine EP Impurity E | Phenylephrine isoquinolinone analog | Phenylephrine Impurity C | Phenylephrine Impurity-40 | N-acetylphenylephrine | Phenylephrine Impurity 12 | Phenylephrine Impurity-39 | Phenylephrine related compound F | Phenylephrine Impurity 26 | Phenylephrine EP Impurity C TFA salt | Phenylephrine Impurity 5 | Phenylephrine Impurity 20 | Phenylephrine isoquinoline 4,6-diol analog | rac 2-Bromo Phenylephrine Hydrochloride | Phenylephrine maleic acid adduct | Phenylephrine Impurity 29 | Phenylephrine Impurity 38 |