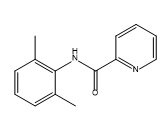
Bupivacaine EP Impurity A
| Product Name | Bupivacaine EP Impurity A |
|---|---|
| Alternate Names | Bupivacaine Impurities, Impurities of Bupivacaine |
| CAT No. | CS-T-39949 |
| CAS No. | 39627-98-0 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 226.27 g/mol |
| Mol. For. | C₁₄H₁₄N₂O |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Bupivacaine |
| Canonical Smiles | CC1=C(C(=CC=C1)C)NC(=O)C2=CC=CC=N2 |
| InchIKey | SHZDEASIMRREIQ-UHFFFAOYSA-N |
| Inchl | InChI=1S/C14H14N2O/c1-10-6-5-7-11(2)13(10)16-14(17)12-8-3-4-9-15-12/h3-9H,1-2H3,(H,16,17) |
| IUPAC | N-(2,6-dimethylphenyl)pyridine-2-carboxamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Bupivacaine EP Impurity A is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard or impurity standard during the analysis of Bupivacaine, which is a local anaesthetic drug that is used to numb specific areas of the body during surgery or other medical procedures.
Bupivacaine EP Impurity A is a white to off-white crystalline powder that is soluble in water and other organic solvents. Its chemical formula is C13H20N2O2 and its molecular weight is 240.31 g/mol. It is also known by its systematic name, (2S)-1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide.
In terms of its usage, Bupivacaine EP Impurity A is primarily used as a reference standard during the analysis of Bupivacaine. It is also used as an impurity standard to ensure the purity and quality of Bupivacaine in pharmaceutical formulations. This compound is generally used as a comparison tool to determine the purity and content of Bupivacaine present in various pharmaceutical products.
In conclusion, Bupivacaine EP Impurity A is an important chemical compound used in the pharmaceutical industry as a reference standard or impurity standard during the analysis of Bupivacaine. Its usage ensures the purity and quality of Bupivacaine in pharmaceutical formulations.
Get an Instant Quote
Related Compounds
Bupivacaine EP Impurity B | Bupivacaine EP Impurity C | Bupivacaine EP Impurity B | Bupivacaine EP Impurity E | Bupivacaine EP Impurity D |