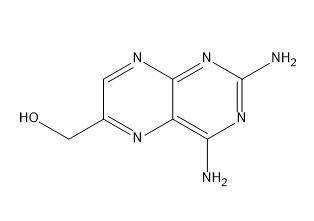
Methotrexate EP Impurity A
| Product Name | Methotrexate EP Impurity A |
|---|---|
| Alternate Names | Methotrexate Impurities, Impurities of Methotrexate |
| CAT No. | CS-T-40938 |
| CAS No. | 945-24-4 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 192.18 g/mol |
| Mol. For. | C₇H₈N₆O |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Methotrexate |
| Therapeutic | Immunosuppressants |
| Smileys | C1=C(N=C2C(=NC(=NC2=N1)N)N)CO |
| Canonical Smiles | C1=C(N=C2C(=NC(=NC2=N1)N)N)CO |
| InchIKey | CYNARAWTVHQHDI-UHFFFAOYSA-N |
| Inchl | InChI=1S/C7H8N6O/c8-5-4-6(13-7(9)12-5)10-1-3(2-14)11-4/h1,14H,2H2,(H4,8,9,10,12,13) |
| IUPAC | (2,4-diaminopteridin-6-yl)methanol |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Methotrexate EP Impurity A is a chemical compound that is commonly used in the pharmaceutical industry. It is classified as an impurity, which means that it is a byproduct of the manufacturing process of methotrexate, a medication used to treat various types of cancer, rheumatoid arthritis, and psoriasis.
The chemical name of Methotrexate EP Impurity A is (2S)-2-[(4-{[(2,4-diaminopteridin-6-yl)methyl]amino}benzoyl)amino]pentanedioic acid. It is an organic compound that is soluble in water and has a molecular weight of 474.47 g/mol.
The primary use of Methotrexate EP Impurity A is as a reference standard for analytical purposes. It is commonly used in the development and validation of analytical methods for the detection and quantification of impurities in methotrexate formulations.
In terms of safety, Methotrexate EP Impurity A is considered to be relatively low-risk. However, like all chemicals, it should be handled with care and stored appropriately to prevent any potential hazards.
Overall, Methotrexate EP Impurity A plays an important role in the pharmaceutical industry by providing a reliable reference standard for the analysis of methotrexate formulations. Its chemical properties and usage make it a valuable tool for ensuring the quality and safety of medications used to treat cancer and other serious medical conditions.
Get an Instant Quote
Related Compounds
Methotrexate - Impurity E (HCl Salt) | Methotrexate EP Impurity B Disodium salt | N-Nitroso Methotrexate EP Impurity-L (2) | N-Dinitroso Methotrexate Impurity 2 | Methotrexate EP Impurity I | Methotrexate EP Impurity J | N-nitroso Methotrexate Ethylester impurity | N-Desmethyl Methotrexate dinitroso impurity | Methotrexate Nitroso Impurity 1 | N-Nitroso Methotrexate EP Impurity-B | Methotrexate EP Impurity B (MonoHydrate) | N-nitroso Methotrexate Impurity 2 | Methotrexate EP Impurity E | Methotrexate EP Impurity L | Methotrexate EP Impurity C | Methotrexate EP Impurity G | Methotrexate Nitroso Impurity 1 | Methotrexate diethyl ester | Methotrexate EP Impurity D | R-Methotrexate di sodium | Methotrexate EP Impurity B | Methotrexate EP Impurity H | N-Nitroso Methotrexate EP Impurity-L (1) | N, N-Dinitroso Methotrexate EP Impurity L | Methotrexate Dimethylamide | Methotrexate EP Impurity F |