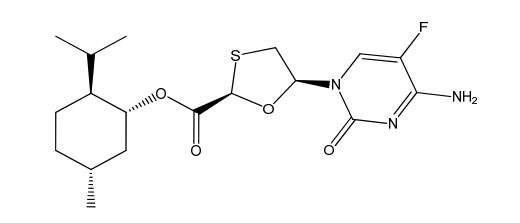
Emtricitabine Impurity
| Product Name | Emtricitabine Impurity |
|---|---|
| Alternate Names | Emtricitabine Impurities, Impurities of Emtricitabine |
| CAT No. | CS-T-47837 |
| CAS No. | 764659-72-5 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 399.48 g/mol |
| Mol. For. | C₁₈H₂₆FN₃O₄S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Emtricitabine |
| Purity | 95% |
| Therapeutic | Antiretroviral / Anti-HIV |
| Smileys | O=C1N([C@H]2O[C@@H](C(O[C@H](C[C@H](C)CC3)[C@@H]3C(C)C)=O)SC2)C=C(F)C(N)=N1 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Emtricitabine Impurity is a chemical compound that is commonly used as a reference material in pharmaceutical research and development. It is a structural isomer of Emtricitabine, which is an antiviral drug that is used to treat HIV and hepatitis B virus infections. Emtricitabine Impurity is a byproduct that is formed during the synthesis of Emtricitabine and is often present in small amounts in finished drug products.
Chemically, Emtricitabine Impurity is a pyrimidine nucleoside that contains a cytosine base and a ribose sugar moiety. It is not active against HIV or hepatitis B virus and does not have any therapeutic use. However, it is used as a reference standard to identify and quantify Emtricitabine in drug formulations and biological samples.
The analytical methods used to detect Emtricitabine Impurity typically involve chromatography techniques such as high-performance liquid chromatography (HPLC) or gas chromatography (GC). The purity of Emtricitabine is determined by comparing the amount of Emtricitabine Impurity present in the sample to the amount of Emtricitabine. The limit of Emtricitabine Impurity allowed in Emtricitabine drug products is typically set at 0.1% or lower.
In conclusion, Emtricitabine Impurity is an important reference material used in the pharmaceutical industry to ensure the quality and purity of Emtricitabine drug products. Its chemical structure and properties are well characterized, and it is used in analytical methods to quantify the amount of Emtricitabine present in drug formulations and biological samples.
Get an Instant Quote
Related Compounds
Desamino Emtricitabine | Emtricitabine lactose adduct | Emtricitabine Impurity 8 | Emtricitabine 5-fluorouracil analog | Trans-cyclo-Emtricitabine | Emtricitabine Glycosamine | Emtricitabine Racemic Mixture | Emtricitabine+Tenofovir FT4 | Emtricitabine+Tenofovir FT1 | Emtricitabine+Tenofovir FT5 | Emtricitabine F6 Impurity | N-Acetyl O-Benzoyl 5-Epi Emtricitabine | Emtricitabine diastereomer(mixture) | Emtricitabine Menthyl Ester(2S,5R Isomer) | Emtricitabine methyl ester | Emtricitabine+Tenofovir FT6 | Emtricitabine Tenofovir Monosoproxil Dimer | Emtricitabine+Tenofovir FT3 | Emtricitabine Impurity 9 | Cyclic Diastereomeric Impurity Isomer-2 | ent-Emtricitabine | conjugate Impurity of Tenofovir+Emtricitabine | Emtricitabine Dimer Impurity | Emtricitabine+Des-Phenol Tenofovir Alfenamide Mixed Dimer | Emtricitabine+Tenofovir FT2 | Emtricitabine dioxolane | Emtricitabine FTU Impurity | Emtricitabine Disulfide | Emtricitabine Tenofovir Disoproxil Dimer | Emtricitabine Diastereomer | Cis-cyclo-Emtricitabine | Emtricitabine (S)-sulphoxide | Emtricitabine Impurity 6 | Emtricitabine 6-Disulfide | Emtricitabine Impurity 11 | Emtricitabine (R)-sulphoxide |