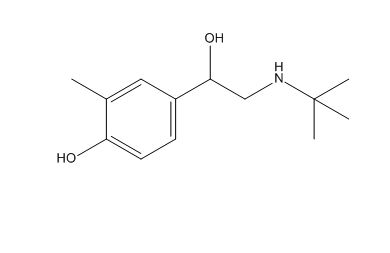
Salbutamol EP Impurity C
| Product Name | Salbutamol EP Impurity C |
|---|---|
| Alternate Names | Salbutamol Impurities, Impurities of Salbutamol |
| CAT No. | CS-T-51812 |
| CAS No. | 18910-68-4 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 223.31 g/mol |
| Mol. For. | C₁₃H₂₁NO₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Salbutamol |
| Purity | 95% |
| Smileys | CC1=C(C=CC(=C1)C(CNC(C)(C)C)O)O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Salbutamol EP Impurity C is a chemical compound that is used as an impurity standard in the pharmaceutical industry. It is a naturally occurring impurity that is commonly found in Salbutamol, a medication used to treat asthma and other respiratory conditions. The presence of this impurity in Salbutamol can have adverse effects on patients, which is why it is important to monitor its levels during the drug manufacturing process.
Salbutamol EP Impurity C has a molecular formula of C12H17NO2 and a molecular weight of 207.27 g/mol. It is a white to off-white powder that is soluble in water, ethanol, and methanol. This impurity is classified as a beta-adrenergic agonist, which means it binds to beta-adrenergic receptors in the body and stimulates the sympathetic nervous system. This can lead to an increase in heart rate, blood pressure, and bronchodilation.
To ensure the safety and efficacy of Salbutamol, pharmaceutical companies must test for the presence of Salbutamol EP Impurity C during the drug manufacturing process. The impurity standard is used to set a limit on the amount of impurity that is allowed in the medication, ensuring that it is safe for patients to use. Overall, Salbutamol EP Impurity C plays a critical role in maintaining the quality and safety of Salbutamol and other beta-adrenergic agonist medications.
Get an Instant Quote
Related Compounds
Salbutamol Glyoxal Impurity | N-Benzyl Salbutamol Hydrochloride | Salbutamol EP Impurity G | Salbutamol Impurity K | Salbutamol EP Impurity I | Salbutamol EP Impurity H | Salbutamol Impurity P | Salbutamol EP Impurity A | Salbutamol Impurity O | Salbutamol Impurity L | Salbutamol EP Impurity B | Salbutamol Impurity N Acetate Salt | Colterol | Salbutamol Related Compound 1 | Salbutamol EP Impurity K (hemihydrate) | Salbutamol EP Impurity E | Salbutamol diethyl ether | Salbutamol Acetonide Methyl Ether | Levalbuterol Related Compound E Hydrochloride | Salbutamol EP Impurity D Sulfate salt | Salbutamol EP Impurity D | Salbutamol Acetonide | Salbutamol EP Impurity L Hydrochloride |