Omeprazole EP impurity F & G Mixture
| Product Name | Omeprazole EP impurity F & G Mixture |
|---|---|
| Alternate Names | Omeprazole Impurities, Impurities of Omeprazole |
| CAT No. | CS-T-57691 |
| CAS No. | 125656-82-8+125656-83-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 311.36+311.36 g/mol |
| Mol. For. | C₁₆H₁₃N₃O₂S+C₁₆H₁₃N₃O₂S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Omeprazole |
| Therapeutic | Anti ulcer |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Omeprazole is a proton-pump inhibitor widely used in the treatment of gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), and other related disorders. However, during the manufacturing process, impurities may be formed, which can affect the safety and efficacy of the final product. Omeprazole EP Impurity F & G Mixture is one such impurity that is commonly found in Omeprazole formulations.
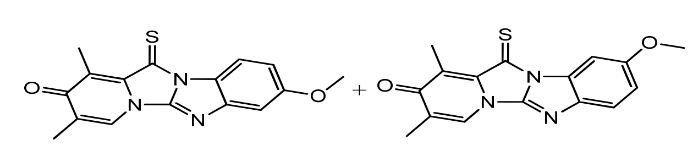
Omeprazole EP Impurity F & G Mixture is a combination of two impurities, namely 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl]-1H-benzimidazole (Impurity F) and 2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl]-5-(1H-1,2,4-triazol-1-yl) -1H-benzimidazole (Impurity G). These impurities are formed during the manufacturing process due to the reaction of Omeprazole with other chemicals.
The usage of Omeprazole EP Impurity F & G Mixture is primarily for research and development purposes. It is used as a reference standard to identify and quantify the presence of these impurities in Omeprazole formulations. The chemical information of Omeprazole EP Impurity F & G Mixture includes its molecular weight, molecular formula, and structural formula.
In conclusion, Omeprazole EP Impurity F & G Mixture is an important reference material used by researchers and manufacturers to ensure the quality and safety of Omeprazole formulations.
Get an Instant Quote
Related Compounds
O,O-Didesmethyl Omeprazole | N-Methyl Esomeprazole Isomer-1 | Omeprazole D13 | Omeprazole Impurity 24 | Omeprazole EP Impurity G | N-methyl Omeprazole | Omeprazole Impurity 25 | Omeprazole Impurity 53 | Bis-Desmethoxy Omeprazole Sulfide | Omeprazole impurity D | o-Toluoyl-5-hydroxy Omeprazole Sulfide | Omeprazole Impurity 79 | N-Nitroso Omeprazole Sulfide | Omeprazole Impurity 8 | Omeprazole Impurity 30 | Desulfoxide 4-Demethyl Omeprazole | Omeprazole Related Compound 7 | Omeprazole O-hydrogen sulfite | Omeprazole impurity (DIMER Mixture) | Omeprazole Impurity 63 | Omeprazole EP Impurity C | Omeprazole-N-(S)-camphorsulfonamide | N-(4-Methoxy-3,5-dimethyl-2-pyridinyl)methyl Omeprazole | Omeprazole Related Compound 5 | Omeprazole Impurity 60 | Omeprazole D15 | Omeprazole Acid Methyl Ester Sulfide | Omeprazole sulfide 5-carboxylic acid | Omeprazole Sulfide N1-Methyl 5-Methoxy Analog | N-Nitroso Omeprazole EP Impurity I | Omeprazole Isomer-2 5-Methoxy -1-[(4-methoxy-3,5-dimethyl-2-pyridinyl)]-0[2-(4-methoxy-3,5-dimethyl -2-pyridinyl)methyl]-sulfanyl]-1H-banzimidole | Omeprazole dibenzimidazole Impurity | N-Methyl Omeprazole (Mixture of isomers with the methylated nitrogens of imidazole) | Omeprazole EP impurity B | Omeprazole EP Impurity A | Omeprazole EP impurity I | Omeprazole Hydrolysis Impurity | Omeprazole EP Impurity F | N-Nitroso Omeprazole sodium | Omeprazole Impurity- K |