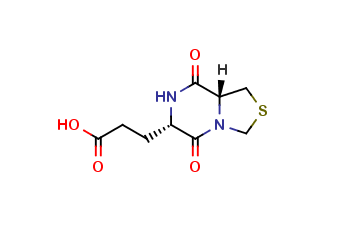
Pidotimod impurity X
| Product Name | Pidotimod impurity X |
|---|---|
| Alternate Names | Pidotimod Impurities, Impurities of Pidotimod |
| CAT No. | CS-T-60581 |
| CAS No. | 161771-75-1 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | Not Available |
| Mol. For. | C9H12N2O4S |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Pidotimod |
| Smileys | O=C(C(N1)CCC(O)=O)N(CSC2)C2C1=O |
| Canonical Smiles | C1C2C(=O)NC(C(=O)N2CS1)CCC(=O)O |
| InchIKey | SIHJCGBZTATYLB-UHFFFAOYSA-N |
| Inchl | InChI=1S/C9H12N2O4S/c12-7(13)2-1-5-9(15)11-4-16-3-6(11)8(14)10-5/h5-6H,1-4H2,(H,10,14)(H,12,13) |
| IUPAC | 3-(5,8-dioxo-3,6,7,8a-tetrahydro-1H-[1,3]thiazolo[3,4-a]pyrazin-6-yl)propanoic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Unfortunately, as an AI language model, I do not have access to specific information on Pidotimod impurity X. Each impurity can have its own unique chemical structure and properties, therefore the usage and chemical information of Pidotimod impurity X may vary.
In general, impurities in pharmaceuticals are unwanted substances that can arise from the manufacturing process or storage conditions. They can potentially affect the safety and efficacy of the drug product. It is important to identify, characterize and control impurities through various analytical techniques and quality control measures.
Chemical information on Pidotimod impurity X may include its molecular formula, weight, melting point and solubility. The usage of the impurity can depend on its properties and the specific drug product it is associated with. It may require further investigation and evaluation to determine the impact on drug potency or stability.
Overall, the control and management of impurities in pharmaceuticals is critical to ensure the safety and efficacy of the drug product.
Get an Instant Quote
Related Compounds
Pidotimod impurity-34 | Pidotimod impurity C | Pidotimod Methyl Ester | Pidotimod impurity Y | Pidotimod impurity: Thiazolidine Carboxylic acid | Pidotimod Ethyl Ester | Pidotimod Impurity 37 | Pidotimod (2S,5R)-3.6-diketopiperazine derivative | Pidotimod Thioproline dimer impurity | Pidotimod impurity E | Pidotimod impurity-26 | Pidotimod Glutamic acid dimer impurity | Pidotimod impurity D | Pidotimod impurity A | Pidotimod impurity B | Pidotimod Impurity 11 | Pidotimod (R,R)-Thioproline dimer impurity | Pidotimod sulfoxide | Pidotimod Glutamic acid derivative | Pidotimod Impurity 10 | Pidotimod imp at RT 27 min |