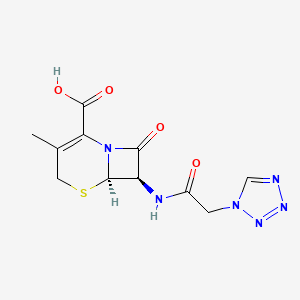
Cefazolin EP Impurity C
| Product Name | Cefazolin EP Impurity C |
|---|---|
| Alternate Names | Cefazolin Impurities, Impurities of Cefazolin |
| CAT No. | CS-ZG-76801 |
| CAS No. | 56842-77-4 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 324.32 g/mol |
| Mol. For. | C₁₁H₁₂N₆O₄S |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Cefazolin |
| Smileys | CC1=C(N2C(C(C2=O)NC(=O)CN3C=NN=N3)SC1)C(=O)O |
| Canonical Smiles | CC1=C(N2C(C(C2=O)NC(=O)CN3C=NN=N3)SC1)C(=O)O |
| InchIKey | DGYURGWZOSQCFG-GMSGAONNSA-N |
| Inchl | InChI=1S/C11H12N6O4S/c1-5-3-22-10-7(9(19)17(10)8(5)11(20)21)13-6(18)2-16-4-12-14-15-16/h4,7,10H,2-3H2,1H3,(H,13,18)(H,20,21)/t7-,10-/m1/s1 |
| IUPAC | (6R,7R)-3-methyl-8-oxo-7-[[2-(tetrazol-1-yl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Cefazolin EP Impurity C, also known as 5-Methyl-2-phenyl-1,3-oxazol-4(5H)-one, is a synthetic organic compound that is used as a reference standard and impurity marker in the analysis of Cefazolin, a widely used antibiotic. Cefazolin is a first-generation cephalosporin antibiotic that is commonly used to treat bacterial infections, including respiratory tract infections, skin infections, and urinary tract infections.
Cefazolin EP Impurity C is a white to off-white powder that is sparingly soluble in water and soluble in most organic solvents. Its chemical formula is C10H9NO2 and it has a molecular weight of 175.18 g/mol. The compound is typically used as a reference standard in quality control and analytical testing of Cefazolin and its related compounds.
In terms of safety and handling, Cefazolin EP Impurity C should be handled with caution, as it may cause irritation to the eyes, skin, and respiratory tract. It should be stored in a cool, dry place and kept away from heat, open flames, and oxidizing agents.
Overall, Cefazolin EP Impurity C plays an important role in the analysis and quality control of Cefazolin and its related compounds, helping to ensure that these medications are safe and effective for patients.
Get an Instant Quote
Related Compounds
Cefazolin EP Impurity B | Cefazolin EP Impurity I | Cefazolin EP Impurity L | Cefazolin EP Impurity C | Cefazolin EP Impurity A | Cefazolin EP Impurity D sodium salt | Cefazolin EP Impurity J disodium salt | Cefazolin EP Impurity J | Cefazolin EP Impurity G (Triethylamine salt) | Cefazolin EP Impurity K | Cefazolin EP Impurity H | Cefazolin EP Impurity F | Cefazolin EP Impurity E | Cefazolin EP Impurity G | Cefazolin Related Compound D (Cefazolin open-ring lactone) |