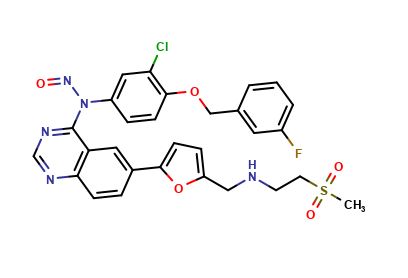
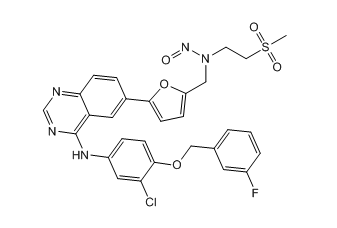
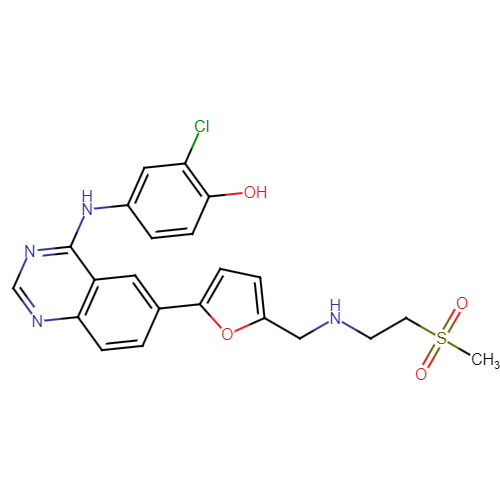
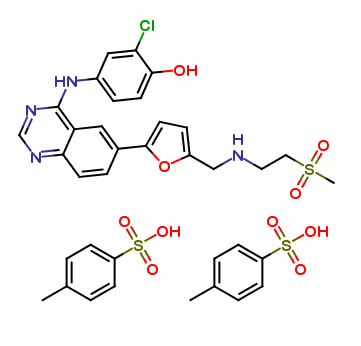
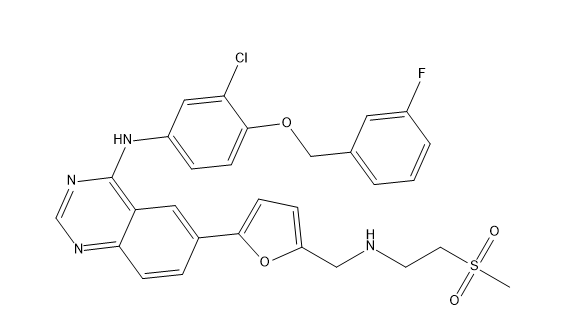
Lapatinib Pharmaceutical Reference Standards
| CAT No. |
: CS-T-93688 |
| Mol F. |
: C29H26ClFN4O4S |
| Mol W. |
:
581.06 g/mol
|
| Cas No. |
: 1393112-45-2 |
| Stock |
: Enquire |
| CAT No. |
: CS-T-88581 |
| Mol F. |
: C₂₆H₁₉ClFN₃O₃ |
| Mol W. |
:
475.90 g/mol
|
| Cas No. |
: 320337-48-2 |
| Stock |
: IN-Stock |
| CAT No. |
: CS-T-88388 |
| Mol F. |
: C29H27FN4O4S |
| Mol W. |
:
546.6 g/mol
|
| Cas No. |
: 633370-23-7 |
| Stock |
: Enquire |
| CAT No. |
: CS-O-48735 |
| Mol F. |
: C29H24ClFN6O6S |
| Mol W. |
:
639.05 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
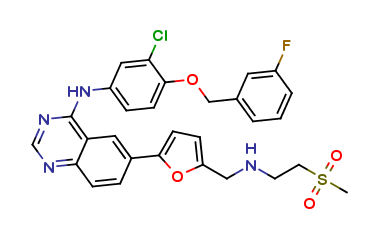
LAPATINIB API STANDARDS, API STANDARDS
Lapatinib
| CAT No. |
: CS-O-01724 |
| Mol F. |
: C₂₉H₂₆ClFN₄O₄S |
| Mol W. |
:
581.06 g/mol
|
| Cas No. |
: 231277-92-2 |
| Stock |
: IN-Stock |
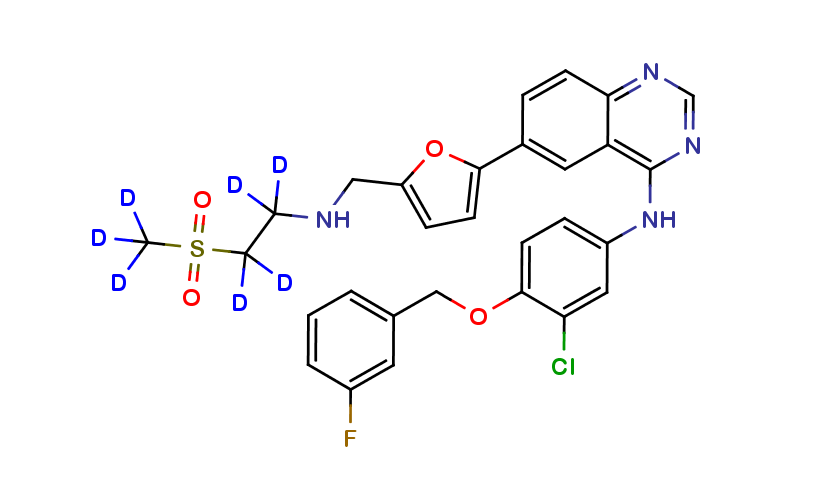
LAPATINIB STABLE ISOTOPES, STABLE ISOTOPES
Lapatinib 13C D7
| CAT No. |
: CS-O-02791 |
| Mol F. |
: Not Available |
| Mol W. |
:
Not Available
|
| Cas No. |
: 1210608-87-9 |
| Stock |
: Enquire |
| CAT No. |
: CS-P-07007 |
| Mol F. |
: Not Available |
| Mol W. |
:
Not Available
|
| Cas No. |
: 1026818-86-9 |
| Stock |
: Enquire |
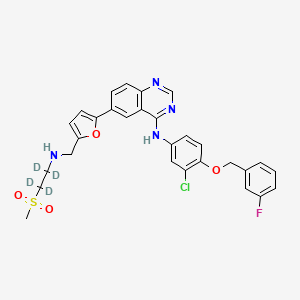
LAPATINIB STABLE ISOTOPES, STABLE ISOTOPES
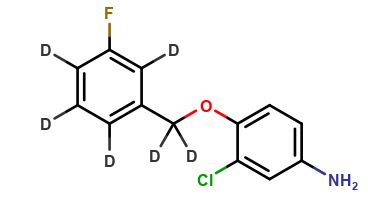
Lapatinib D4
| CAT No. |
: CS-O-32802 |
| Mol F. |
: C₂₉H₂₂D₄ClFN₄O₄S |
| Mol W. |
:
585.08 g/mol
|
| Cas No. |
: 1184263-99-7 |
| Stock |
: IN-Stock |
| CAT No. |
: CS-O-34908 |
| Mol F. |
: C41H34D4ClFN4O10S3 |
| Mol W. |
:
901.43 mol/g g/mol
|
| Cas No. |
: 388082-78-8 (unlabeled) |
| Stock |
: Enquire |
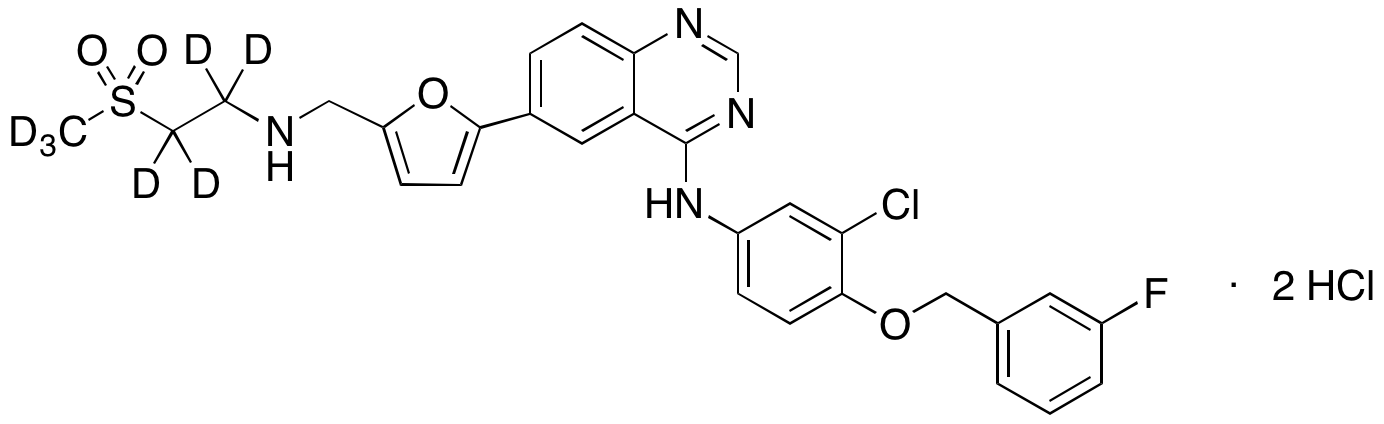
LAPATINIB STABLE ISOTOPES, STABLE ISOTOPES
Lapatinib D6
| CAT No. |
: CS-EO-02540 |
| Mol F. |
: C13H5D6ClFNO |
| Mol W. |
:
Not Available
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
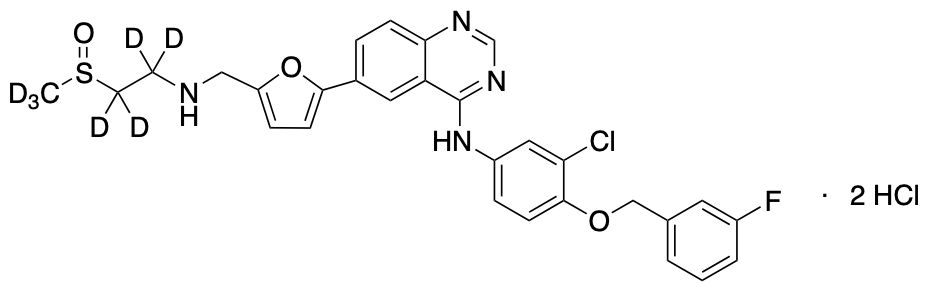
LAPATINIB STABLE ISOTOPES, STABLE ISOTOPES
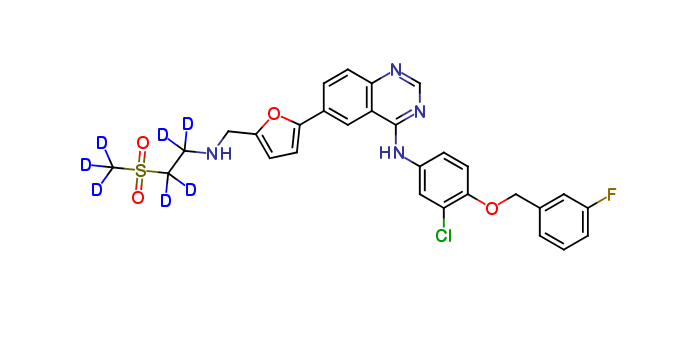
Lapatinib D7
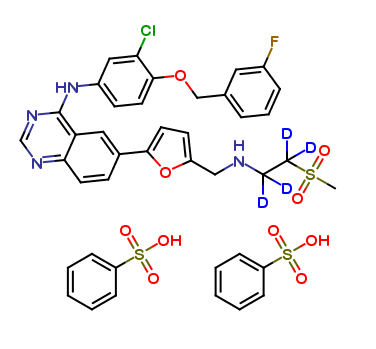
| CAT No. |
: CS-T-93471 |
| Mol F. |
: C29H19D7ClFN4O4S |
| Mol W. |
:
588.1 g/mol
|
| Cas No. |
: 1009307-23-6 |
| Stock |
: Enquire |
LAPATINIB IMPURITIES, IMPURITIES
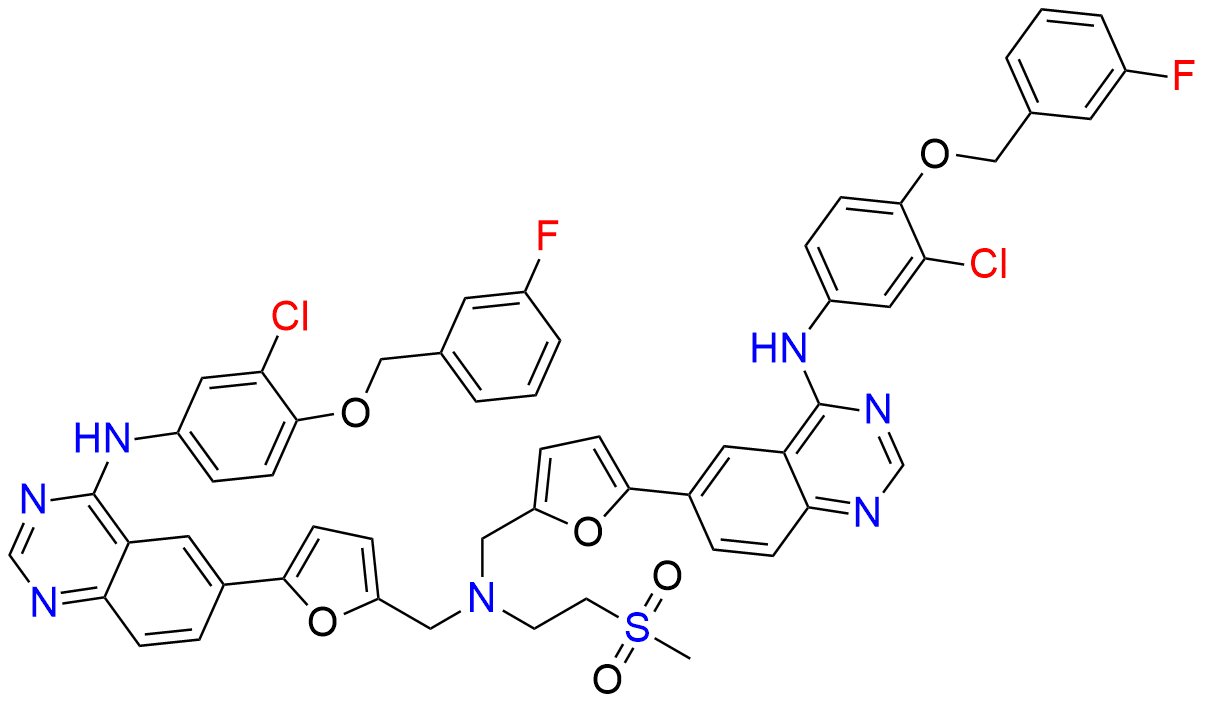
Lapatinib Dimer
| CAT No. |
: CS-O-42623 |
| Mol F. |
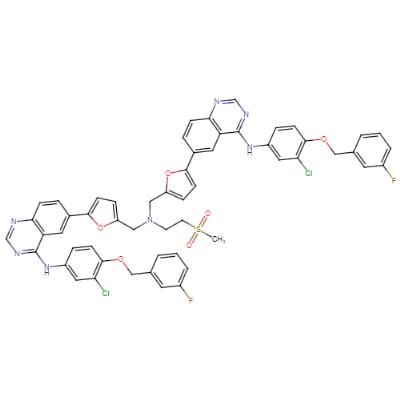
: C55H43Cl2F2N7O6S |
| Mol W. |
:
1038.94 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
| CAT No. |
: CS-M-57890 |
| Mol F. |
: Not Available |
| Mol W. |
:
Not Available
|
| Cas No. |
: 388082-77-7 |
| Stock |
: Enquire |
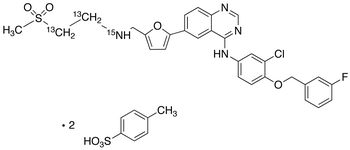
| CAT No. |
: CS-EL-00074 |
| Mol F. |
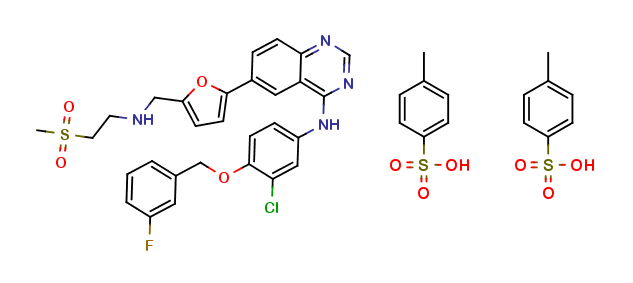
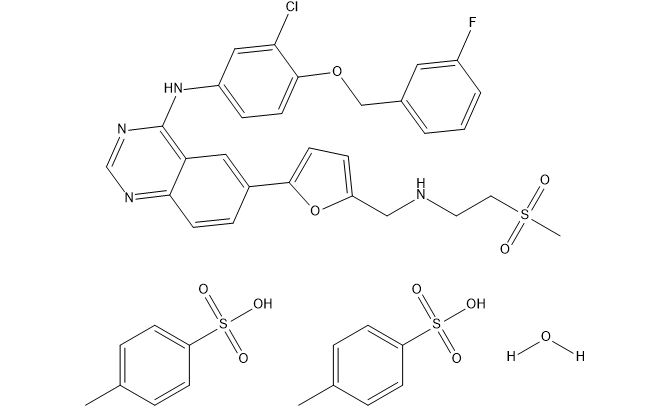
: C29H26ClFN4O4S : 2(C7H8O3S) : H2O |
| Mol W. |
:
581.1 : 2(172.2) : 18.0 g/mol
|
| Cas No. |
: 388082-78-8 |
| Stock |
: IN-Stock |
| CAT No. |
: CS-EO-03637 |
| Mol F. |
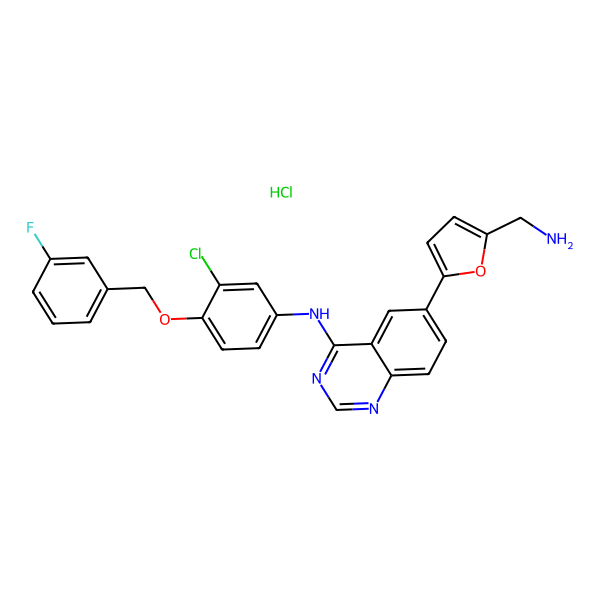
: C26H20ClFN4O2 : HCl |
| Mol W. |
:
474.9 : 36.5 g/mol
|
| Cas No. |
: 697299-82-4 (Freebase) |
| Stock |
: Enquire |
| CAT No. |
: CS-O-43373 |
| Mol F. |
: C55H43Cl2F2N7O6S |
| Mol W. |
:
1038.94 g/mol
|
| Cas No. |
: 2172855-57-9 |
| Stock |
: Enquire |
LAPATINIB STABLE ISOTOPES, STABLE ISOTOPES
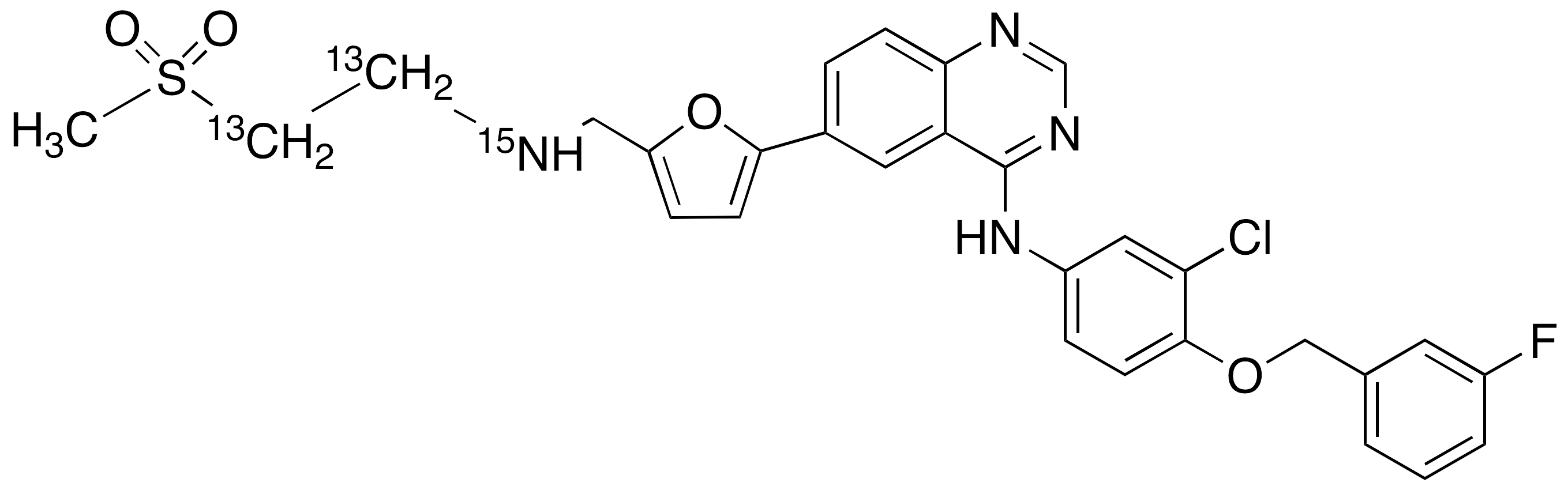
Lapatinib-13C2,15N
| CAT No. |
: CS-T-98820 |
| Mol F. |
: C27¹³C2H26ClFN3¹5NO4S |
| Mol W. |
:
584.04 g/mol
|
| Cas No. |
: 1246819-07-7 |
| Stock |
: Enquire |
| CAT No. |
: CS-T-98821 |
| Mol F. |
: C41¹³C2H42ClFN3¹5NO10S3 |
| Mol W. |
:
928.44 g/mol
|
| Cas No. |
: 1246819-08-8 |
| Stock |
: Enquire |
| CAT No. |
: CS-T-98819 |
| Mol F. |
: C29H21D7Cl3FN4O4S |
| Mol W. |
:
661.02 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
| CAT No. |
: CS-T-100108 |
| Mol F. |
: C29H19D7ClFN4O3S • 2(HCl) |
| Mol W. |
:
572.10 + 2(36.46) g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
| CAT No. |
: CS-T-73116 |
| Mol F. |
: Not Available |
| Mol W. |
:
Not Available
|
| Cas No. |
: 697299-82-4 |
| Stock |
: Enquire |
| CAT No. |
: CS-O-39150 |
| Mol F. |
: C29H25ClFN5O5S |
| Mol W. |
:
610.06 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
| CAT No. |
: CS-O-51177 |
| Mol F. |
: C29H25ClFN5O5S |
| Mol W. |
:
610.06 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
| CAT No. |
: CS-N-05272 |
| Mol F. |
: C22H21ClN4O4S |
| Mol W. |
:
472.94 g/mol
|
| Cas No. |
: 1268997-70-1 |
| Stock |
: Enquire |
| CAT No. |
: CS-T-93781 |
| Mol F. |
: C36H37ClN4O10S3 |
| Mol W. |
:
817.35 g/mol
|
| Cas No. |
: 1268997-70-1 (free base) |
| Stock |
: Enquire |


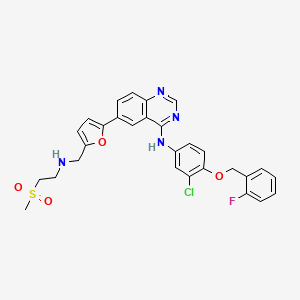
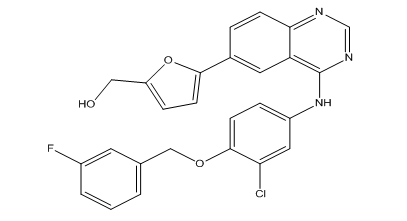
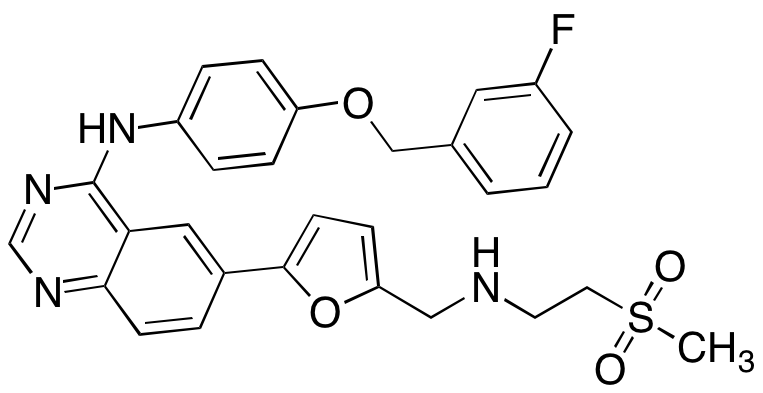
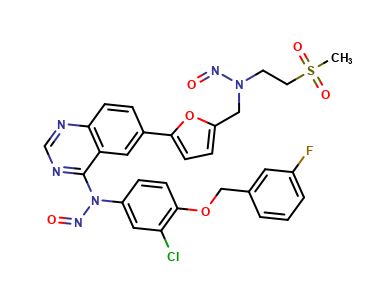
![Lapatinib N-De[2-(methylsulfonyl)ethyl] Lapatinib](https://clearsynth.com/structure/N-De-2--methylsulfonyl-ethyl--Lapatinib.png)