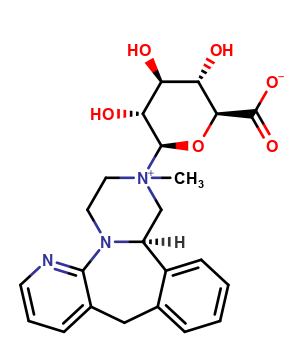
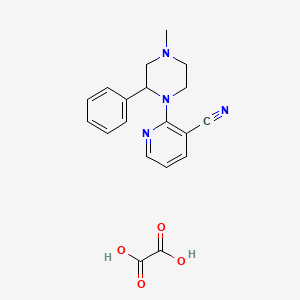
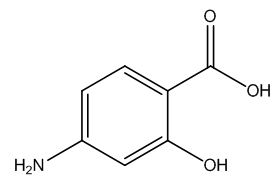
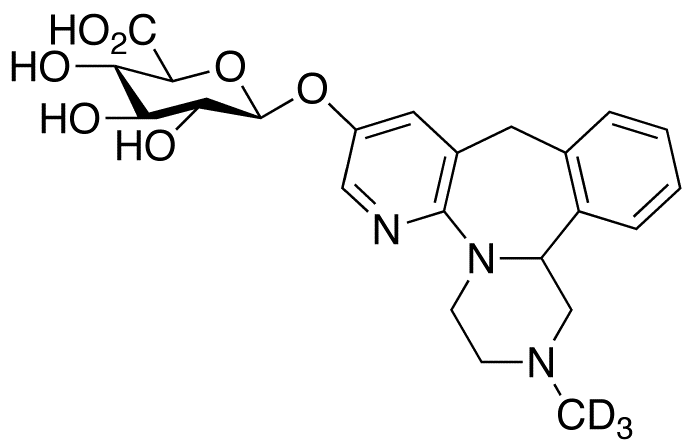
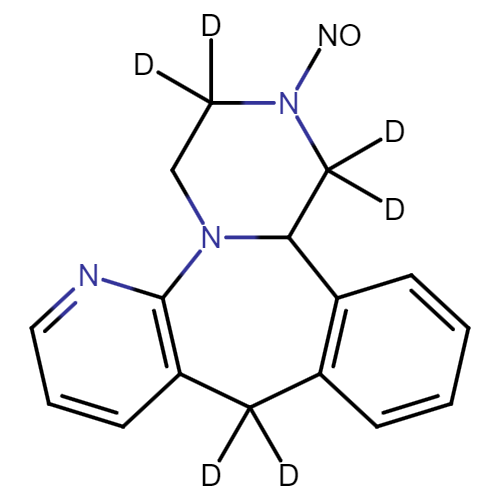
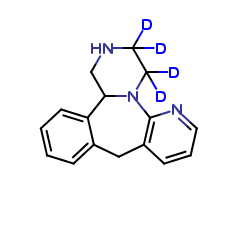
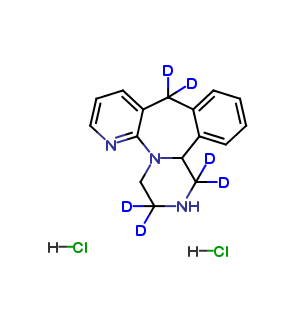
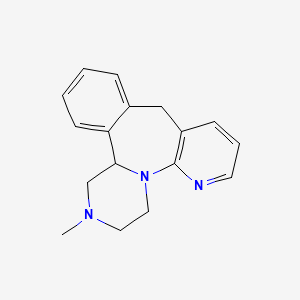
Mirtazapine Pharmaceutical Reference Standards
| CAT No. |
: CS-O-52640 |
| Mol F. |
: C23H27N3O6 |
| Mol W. |
:
441.48 g/mol
|
| Cas No. |
: 205121-67-1 |
| Stock |
: Enquire |
| CAT No. |
: CS-O-40540 |
| Mol F. |
: C17H18N4 : C2H2O4 |
| Mol W. |
:
278.4 : 90.0 g/mol
|
| Cas No. |
: 331815-15-7 |
| Stock |
: IN-Stock |
| CAT No. |
: CS-T-03461 |
| Mol F. |
: C₇H₇NO₃ |
| Mol W. |
:
153.14 g/mol
|
| Cas No. |
: 65-49-6 |
| Stock |
: IN-Stock |
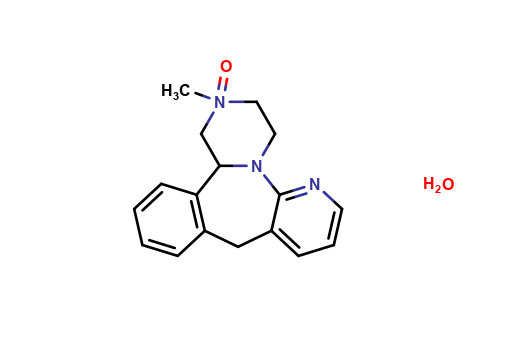
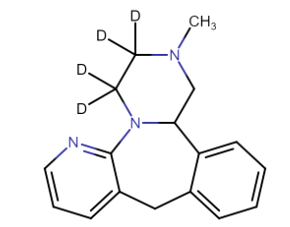
| CAT No. |
: CS-T-98448 |
| Mol F. |
: C23H24D3N3O7 |
| Mol W. |
:
460.49 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
| CAT No. |
: CS-O-42867 |
| Mol F. |
: C16H10D6N4O |
| Mol W. |
:
286.36 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
| CAT No. |
: CS-O-35523 |
| Mol F. |
: C16H13D4N3 |
| Mol W. |
:
255.35 g/mol
|
| Cas No. |
: 1188331-80-7 |
| Stock |
: Enquire |
| CAT No. |
: CS-T-93859 |
| Mol F. |
: C16H13D6Cl2N3 |
| Mol W. |
:
330.29 g/mol
|
| Cas No. |
: 61337-68-6 (unlabeled) |
| Stock |
: Enquire |
MIRTAZAPINE SECONDARY STANDARDS, SECONDARY STANDARDS
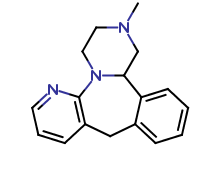
Mirtazapine
| CAT No. |
: CS-ER-03074 |
| Mol F. |
: C₁₇H₁₉N₃ |
| Mol W. |
:
265.35 g/mol
|
| Cas No. |
: 85650-52-8 |
| Stock |
: Enquire |
| CAT No. |
: CS-EG-00873 |
| Mol F. |
: C17H19N3 |
| Mol W. |
:
265.35 g/mol
|
| Cas No. |
: 61337-67-5 |
| Stock |
: Enquire |
MIRTAZAPINE STABLE ISOTOPES, STABLE ISOTOPES
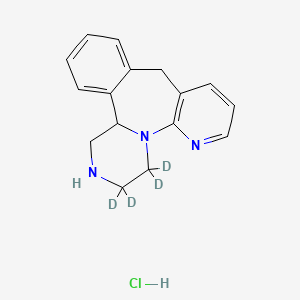
Mirtazapine d4 DIHCl
| CAT No. |
: CS-EO-02471 |
| Mol F. |
: C17H17D4Cl2N3 |
| Mol W. |
:
342.3 g/mol
|
| Cas No. |
: 85650-52-8 unlabeled free base |
| Stock |
: Enquire |
| CAT No. |
: CS-T-58857 |
| Mol F. |
: C₁₇H₁₉N₃O |
| Mol W. |
:
281.35 g/mol
|
| Cas No. |
: 155172-12-6 |
| Stock |
: IN-Stock |
| CAT No. |
: CS-EG-02998 |
| Mol F. |
: C17H19N3 |
| Mol W. |
:
265.35 g/mol
|
| Cas No. |
: 61337-67-5 |
| Stock |
: Enquire |
| CAT No. |
: CS-O-52660 |
| Mol F. |
: C17H21N3O2 |
| Mol W. |
:
299.37 g/mol
|
| Cas No. |
: 2749357-59-1 |
| Stock |
: Enquire |
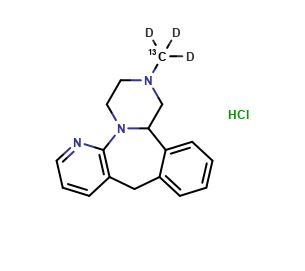
MIRTAZAPINE STABLE ISOTOPES, STABLE ISOTOPES
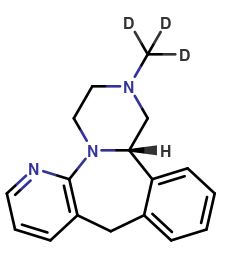
Mirtazapine-13C-D3 HCl
| CAT No. |
: CS-EO-02428 |
| Mol F. |
: C1613CH16D3N3.HCl |
| Mol W. |
:
305.82 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
| CAT No. |
: CS-T-98426 |
| Mol F. |
: C23H25D3N3O6 |
| Mol W. |
:
445.5 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
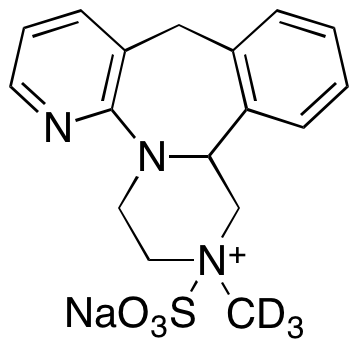
| CAT No. |
: CS-T-102210 |
| Mol F. |
: C17H16D3N3NaO3S |
| Mol W. |
:
371.42 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
MIRTAZAPINE STABLE ISOTOPES, STABLE ISOTOPES
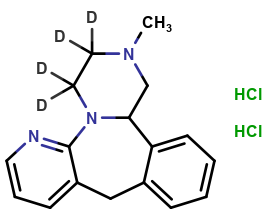
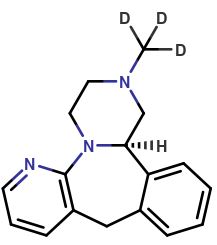
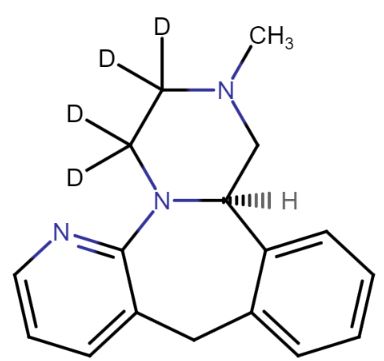
Mirtazapine-d4
| CAT No. |
: CS-T-83650 |
| Mol F. |
: C₁₇H₁₅D₄N₃ |
| Mol W. |
:
269.38 g/mol
|
| Cas No. |
: 1215898-55-7 |
| Stock |
: Enquire |
| CAT No. |
: CS-SN-00053 |
| Mol F. |
: C16H14D4ClN3 |
| Mol W. |
:
291.81 g/mol
|
| Cas No. |
: 1188266-12-7 |
| Stock |
: Enquire |
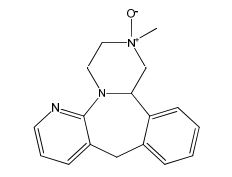
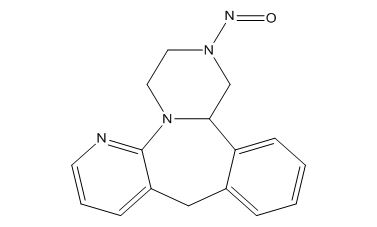
| CAT No. |
: CS-EO-02307 |
| Mol F. |
: C₁₆H₁₆N₄O |
| Mol W. |
:
280.32 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: IN-Stock |
MIRTAZAPINE STABLE ISOTOPES, STABLE ISOTOPES
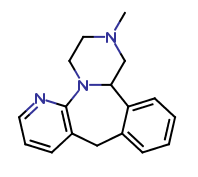
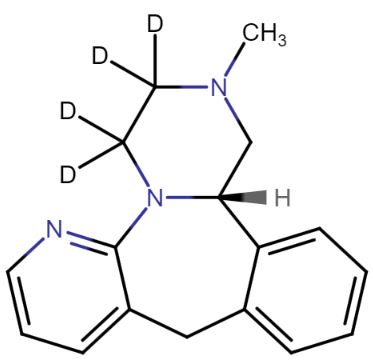
R-Mirtazapine-d3
| CAT No. |
: CS-EO-02548 |
| Mol F. |
: C17H16D3N3 |
| Mol W. |
:
268.4 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
MIRTAZAPINE STABLE ISOTOPES, STABLE ISOTOPES
R-Mirtazapine-d4
| CAT No. |
: CS-O-44260 |
| Mol F. |
: C₁₇H₁₅D₄N₃ |
| Mol W. |
:
269.38 g/mol
|
| Cas No. |
: 61364-37-2 (Unlabelled) |
| Stock |
: Enquire |
MIRTAZAPINE STABLE ISOTOPES, STABLE ISOTOPES
S-Mirtazapine-d3
| CAT No. |
: CS-EO-02549 |
| Mol F. |
: C17H16D3N3 |
| Mol W. |
:
268.4 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
MIRTAZAPINE STABLE ISOTOPES, STABLE ISOTOPES
S-Mirtazapine-d4
| CAT No. |
: CS-O-44261 |
| Mol F. |
: C₁₇H₁₅D₄N₃ |
| Mol W. |
:
269.38 g/mol
|
| Cas No. |
: 61337-87-9 (Unlabelled) |
| Stock |
: Enquire |