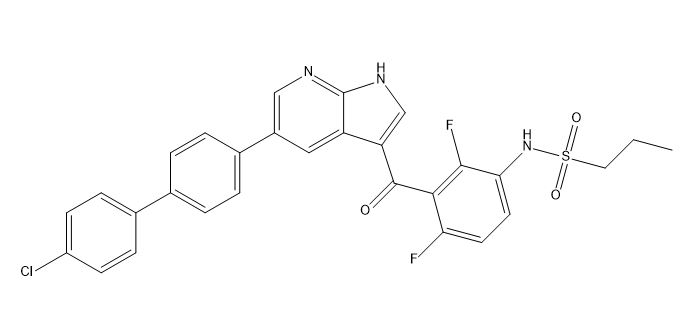
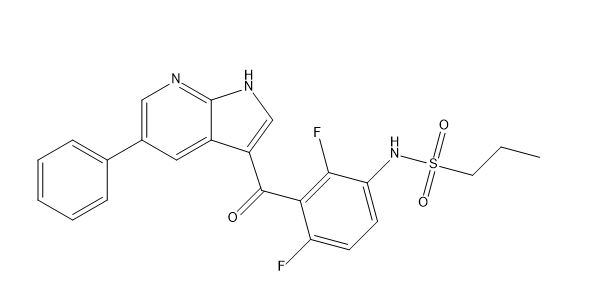
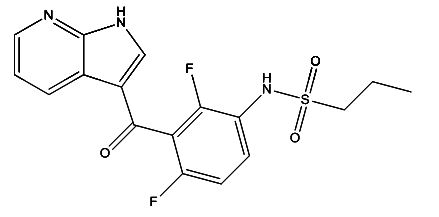
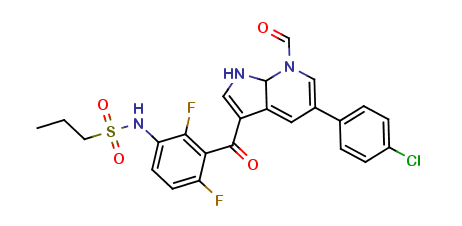
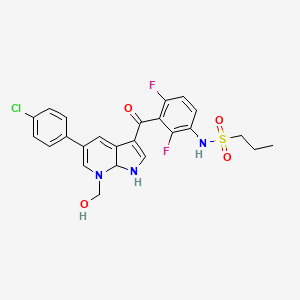
Vemurafenib Impurities and its Related Products
Vemurafenib is a drug used for the treatment of melanoma. However, during the manufacturing process of vemurafenib, impurities may be formed as a result of chemical reactions. These impurities can affect the quality, safety, and efficacy of the drug. Therefore, it is essential to identify and control these impurities to ensure the highest quality of the drug product. Analyzing and identifying these impurities requires sophisticated analytical techniques and expertise in the field of pharmaceutical chemistry.