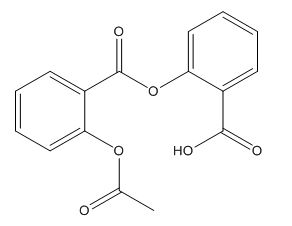
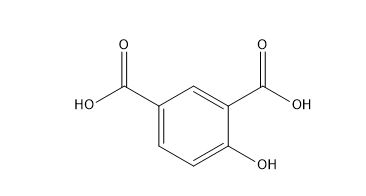
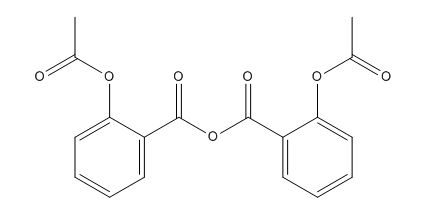
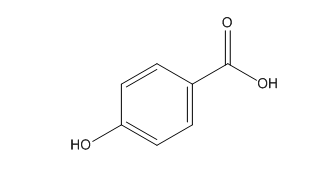
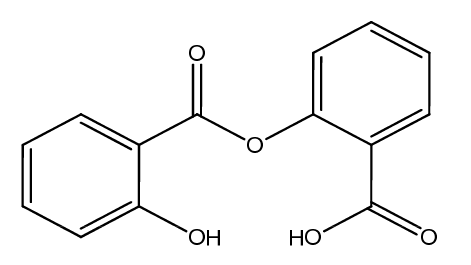
Aspirin Impurities and its Related Products
Aspirin impurities are unwanted substances that can be present in aspirin products. These impurities can be introduced during the manufacturing process or can be present in the raw materials used to produce aspirin. Some common aspirin impurities include salicylic acid, acetic acid, and related compounds. These impurities can have adverse effects on the quality and safety of aspirin products and must be monitored and controlled through strict quality control measures.