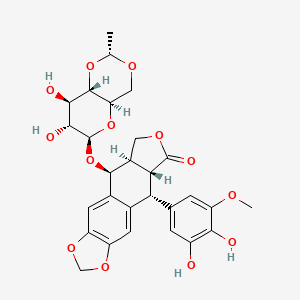
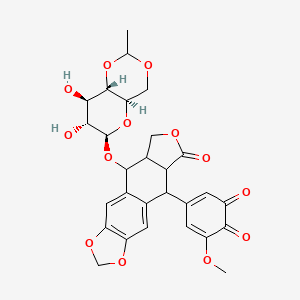
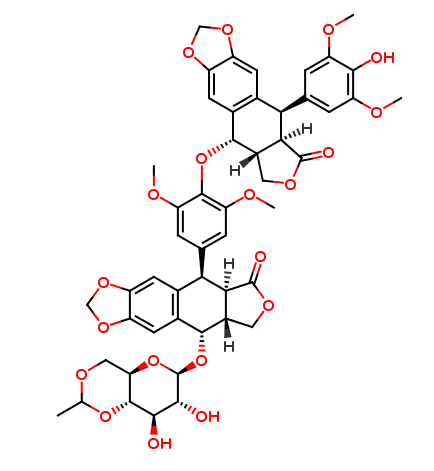
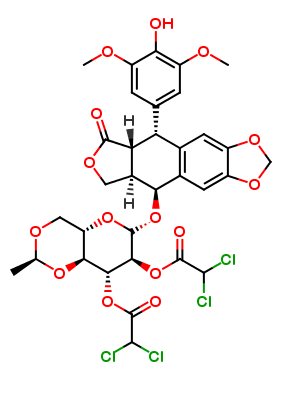
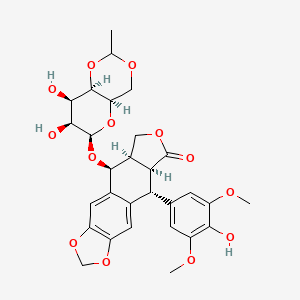
Etoposide Impurities and its Related Products
Etoposide Impurities are the byproducts or contaminants that can be present in the Etoposide drug substance or drug product. These impurities can potentially affect the safety, efficacy, and stability of the drug. Therefore, it is essential to identify, quantify, and control these impurities during the drug development and manufacturing process to ensure the quality of the final product. The most common Etoposide impurities include degradation products, process-related impurities, and residual solvents.