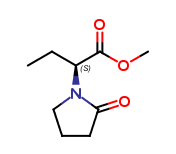
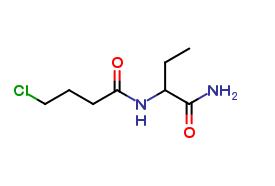
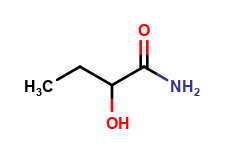
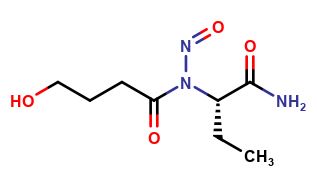
Levetiracetam Impurities and its Related Products
Levetiracetam is a commonly used drug for the treatment of epilepsy. However, during its production and storage, impurities may develop that can affect its potency and safety. These impurities can be organic or inorganic and may arise from the starting materials, manufacturing processes, or storage conditions. The identification and quantification of these impurities are critical to ensure the quality and safety of the drug.