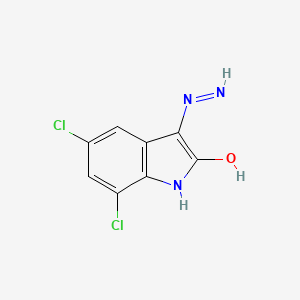
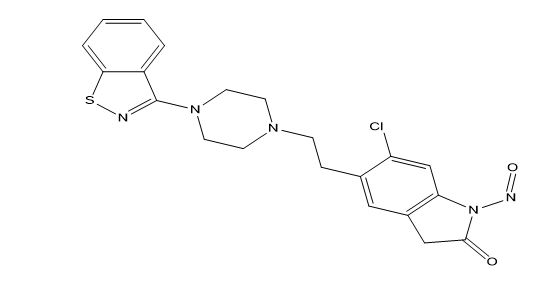
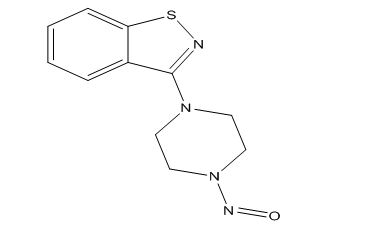
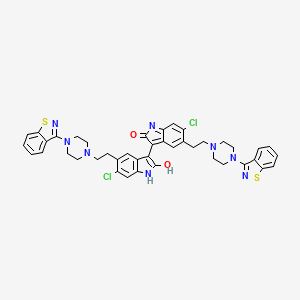
Ziprasidone Impurities and its Related Products
Ziprasidone is an antipsychotic medication used to treat schizophrenia and bipolar disorder. Like all pharmaceuticals, it may contain impurities such as residual solvents, degradation products, and other contaminants. These impurities can affect the drug's efficacy and safety, and therefore it is essential to monitor and control their levels during the manufacturing process. Regulatory authorities impose strict limits on the allowable levels of impurities in pharmaceuticals, including Ziprasidone, to ensure patient safety.