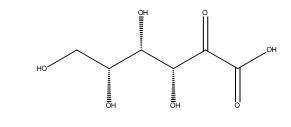
Ascorbic Acid EP Impurity C
| Product Name | Ascorbic Acid EP Impurity C |
|---|---|
| Alternate Names | Ascorbic Acid Impurities, Impurities of Ascorbic Acid |
| CAT No. | CS-T-62791 |
| CAS No. | 16533-48-5 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 194.14 g/mol |
| Mol. For. | C₆H₁₀O₇ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ascorbic Acid |
| Purity | Not less than 90 % |
| Smileys | C(C(C(C(C(=O)C(=O)O)O)O)O)O |
| Canonical Smiles | C(C(C(C(C(=O)C(=O)O)O)O)O)O |
| InchIKey | VBUYCZFBVCCYFD-FLRLBIABSA-N |
| Inchl | InChI=1S/C6H10O7/c7-1-2(8)3(9)4(10)5(11)6(12)13/h2-4,7-10H,1H2,(H,12,13)/t2-,3+,4-/m1/s1 |
| IUPAC | (3R,4S,5R)-3,4,5,6-tetrahydroxy-2-oxohexanoic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ascorbic Acid EP Impurity C, also known as L-threonic acid, is a chemical compound that is commonly used in the pharmaceutical industry as an impurity reference standard. It is a water-soluble organic acid that is structurally similar to vitamin C, also known as ascorbic acid. Ascorbic Acid EP Impurity C is typically used in analytical testing to help identify and quantify the presence of impurities in drug substances and formulations.
Chemically, Ascorbic Acid EP Impurity C is a six-carbon sugar acid that is derived from L-threonine, an essential amino acid. It has a molecular weight of 180.16 g/mol and a chemical formula of C6H10O6. Ascorbic Acid EP Impurity C is stable under normal conditions and has a melting point of approximately 165-170°C.
In pharmaceutical applications, Ascorbic Acid EP Impurity C is used as a reference standard for impurity identification and quantification in drug substances and formulations. It can also be used as a starting material for the synthesis of other organic compounds. Ascorbic Acid EP Impurity C is typically produced through chemical synthesis using threose as a starting material.
Overall, Ascorbic Acid EP Impurity C plays an important role in the pharmaceutical industry, specifically in the analysis and identification of impurities in drug substances and formulations. Its stability and water solubility make it a useful tool for analytical testing and quality control.
Get an Instant Quote
Related Compounds
Ascorbic Acid EP Impurity D | Ascorbic Acid EP Impurity H | Magnesium Ascorbic Acid Phosphate and Ciprofloxacin Hydrochloride | Ferrous Ascorbate | Dehydro-L-(+)-ascorbic acid dimer | Ascorbic Acid EP Impurity A | Ascorbic Acid EP Impurity F | L-Dehydro Ascorbic Acid | Dehydro Ascorbic Acid (threo-2,3-Hexodiulosonic Acid, gama-lactone) | Ascorbic Acid EP Impurity B | Ascorbic Acid Impurity 6 (L-xylo-2-Hexulopyranosonic acid) | Ascorbic Acid EP Impurity G |