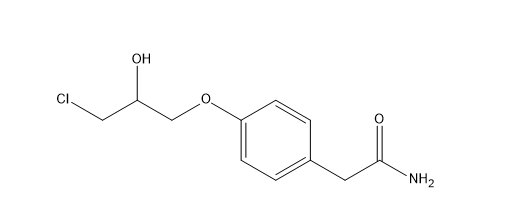
Atenolol EP Impurity D
| Product Name | Atenolol EP Impurity D |
|---|---|
| Alternate Names | Atenolol Impurities, Impurities of Atenolol |
| CAT No. | CS-O-07258 |
| CAS No. | 115538-83-5 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 243.69 g/mol |
| Mol. For. | C₁₁H₁₄ClNO₃ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Atenolol |
| Purity | 95% |
| Therapeutic | Anti-Migraines |
| Smileys | OC(CCl)COC1=CC=C(CC(N)=O)C=C1 |
| Canonical Smiles | C1=CC(=CC=C1CC(=O)N)OCC(CCl)O |
| InchIKey | OQFMSHFNOSFLJU-UHFFFAOYSA-N |
| Inchl | InChI=1S/C11H14ClNO3/c12-6-9(14)7-16-10-3-1-8(2-4-10)5-11(13)15/h1-4,9,14H,5-7H2,(H2,13,15) |
| IUPAC | 2-[4-(3-chloro-2-hydroxypropoxy)phenyl]acetamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Atenolol EP Impurity D is a chemical compound that is used as a reference standard for the analysis and quantification of impurities in atenolol pharmaceutical formulations. It is also used in research and development studies to evaluate the purity and quality of atenolol raw materials.
Chemically, Atenolol EP Impurity D is known as 4-(Acetylamino)-3-chloro-5-(2-methyl-2-propanyl)-2,1,6-benzothiadiazin-7-yl disulfide. It has a molecular formula of C15H19ClN4O2S3 and a molecular weight of 437.98 g/mol.
Atenolol EP Impurity D is a highly stable compound that can withstand various analytical conditions. It is soluble in organic solvents such as methanol, ethanol, and acetonitrile. Its purity is typically measured using high-performance liquid chromatography (HPLC) or gas chromatography (GC).
The presence of impurities in atenolol formulations can affect the efficacy and safety of the drug. Therefore, the use of Atenolol EP Impurity D as a reference standard is essential in ensuring the quality and purity of atenolol products. Its availability in the market has facilitated research and development in the pharmaceutical industry, resulting in the production of safer and more effective atenolol formulations.
Get an Instant Quote
Related Compounds
Atenolol EP Impurity G | Atenolol EP Impurity B | Atenolol EP Impurity C | Atenolol Impurity Standard BP | Atenolol Blocker Acid Impurity | Hydroxyatenolol | Atenolol EP Impurity F | N-FORMYL ATENOLOL | N-Nitroso-Atenolol EP Impurity G | Atenolol EP impurity E | Atenolol Impurity H | Atenolol EP Impurity G | R Atenolol | Atenolol EP Impurity G (sodium salt) | N-Nitroso Atenolol-D7 | N-Nitroso-Atenolol EP Impurity H | Atenolol EP Impurity I |