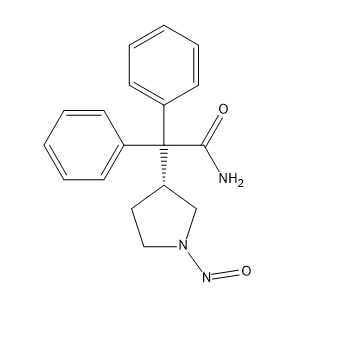
Darifenacin nitroso impurity
Also known as: Darifenacin Nitrosamine Impurities or nitrosamine impurities of Darifenacin| Product Name | Darifenacin nitroso impurity |
|---|---|
| Alternate Names | Darifenacin Impurities, Impurities of Darifenacin |
| CAT No. | CS-O-43262 |
| CAS No. | Not Available |
| Category | Impurities |
| Stock | Enquire |
| Mol. Wt. | 309.36 g/mol |
| Mol. For. | C₁₈H₁₉N₃O₂ |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Darifenacin |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Darifenacin is a medication used to treat urinary incontinence, overactive bladder, and frequent urination. It works by blocking the action of a chemical called acetylcholine, which is responsible for controlling the contraction of bladder muscles. This helps to reduce the frequency of urination and improve bladder control.
However, Darifenacin may contain impurities that can affect its efficacy and safety. One such impurity is the Darifenacin Nitroso Impurity. This impurity is a byproduct of the manufacturing process and can be present in small amounts in Darifenacin formulations.
The chemical structure of Darifenacin Nitroso Impurity is not fully known, but it is believed to be a nitrosamine compound. Nitrosamines are known to be carcinogenic and can cause DNA damage, leading to cancer. Therefore, it is important to monitor the levels of Darifenacin Nitroso Impurity in Darifenacin formulations and ensure that they are within acceptable limits.
To ensure the safety and efficacy of Darifenacin, regulatory authorities such as the FDA require manufacturers to conduct rigorous testing to detect and quantify impurities such as Darifenacin Nitroso Impurity. Manufacturers must also implement measures to minimize the formation of impurities during the manufacturing process. Patients who take Darifenacin should be aware of the potential risks associated with impurities and consult their healthcare provider if they experience any adverse effects.
Get an Instant Quote
Related Compounds
Darifenacin Impurity 11 | Darifenacin 7-Bromoethyl Impurity | Darifenacin Dehydro Impurity | Darifenacin 7-Bromo Analog | Darifenacin Pyrrolidin Carboxylic Acid Impurity | Darifenacin Dimer-1 Impurity | Darifenacin 4-Hydroxy Impurity | Darifenacin Pyrrolidinium Dimer acetonitrile Impurity | Darifenacin Impurity C | Darifenacin Pyrrolidinium Dimer Impurity | Darifenacin 4-Hydroxy Impurity | Darifenacin 3-Hydroxy Impurity | Darifenacin acid | Darifenacin Nitrile Impurity | Darifenacin Vinyl Impurity | Darifenacin Carboxylic Acid Impurity | Darifenacin Nitrile Impurity (HCl) | Darifenacin R-Isomer (HCl) | Darifenacin Dimer-2 Impurity | Darifenacin Open chain | Darifenacin Pyrrolidine Impurity Racemate | Darifenacin Impurity 10 (Tartarate salt) | Darifenacin Oxidized Impurity | Darifenacin Cyano Impurity | Darifenacin Bromo Impurity | Darifenacin Impurity 6 | Darifenacin Cyclic Amide-Impurity E |