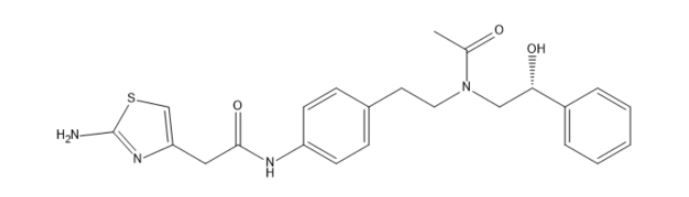
Mirabegron Impurity E
| Product Name | Mirabegron Impurity E |
|---|---|
| Alternate Names | Mirabegron Impurities, Impurities of Mirabegron |
| CAT No. | CS-O-35740 |
| CAS No. | Not Available |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 438.54 g/mol |
| Mol. For. | C₂₃H₂₆N₄O₃S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Mirabegron |
| Smileys | O=C(NC1=CC=C(CCN(C[C@H](O)C2=CC=CC=C2)C(C)=O)C=C1)CC3=CSC(N)=N3 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Mirabegron Impurity E is a chemical compound that is used in the pharmaceutical industry as a reference standard for quality control purposes during the production of Mirabegron, a medication used to treat overactive bladder. The compound is also known by its chemical name, 2-(2-(2-(2,6-dimethylphenoxy)ethoxy)ethoxy)benzamide.
Mirabegron Impurity E is a white to off-white powder that is soluble in organic solvents such as methanol and acetonitrile. It has a molecular weight of 365.47 g/mol and a melting point of 98-102°C. The compound is a derivative of benzamide and contains various functional groups such as ether, amide, and aromatic rings.
The chemical information of Mirabegron Impurity E is important for the pharmaceutical industry as it helps ensure the purity and quality of the final product. It is used as a reference standard during the production process to ensure that the Mirabegron medication meets the required quality standards. Additionally, the compound can also be used in research laboratories for analytical purposes.
In conclusion, Mirabegron Impurity E is an important compound in the pharmaceutical industry, providing a reference standard for quality control and analytical purposes during the production of Mirabegron medication. Its chemical properties and information play a vital role in ensuring the purity and quality of the final product.
Get an Instant Quote
Related Compounds
Mirabegron Deshydroxy Impurity | rac-Mirabegron | Mirabegron Impurity 46 | Dehydroxy Mirabegron Hydrochloride Salt | N-Nitroso Mirabegron Impurity 26 | Mirabegron Impurity 9 | N-Nitroso-Mirabegron (Amide N-Nitroso) | Mirabegron S-Isomer | Mirabegron N-formyl Impurity | Mirabegron Impurity 47 | rac-N-Nitroso Mirabegron | Mirabegron Impurity 13 | Mirabegron Impurity 34 | Mirabegron Impurity 18 | Mirabegron M14 | Mirabegron Impurity 2 | ortho-Mirabegron | Mirabegron Impurity 2 | Mirabegron Impurity 26 | Mirabegron M11 | Mirabegron Impurity 17 | N-Nitroso Mirabegron Impurity | Nitro Mirabegron Impurity 33 | Mirabegron Impurity 11 | Mirabegron Impurity 5 | Mirabegron Impurity 33 | Mirabegron Impurity 4 | Mirabegron Impurity 48 | N-Nitroso Mirabegron Impurity 6 | Mirabegron Impurity 16 | Mirabegron Impurity C | Mirabegron Impurity 3 | Mirabegron Impurity 12 | Mirabegron Impurity 30 | Mirabegron Impurity 14 | Mirabegron Impurity 31 | Mirabegron Impurity 2 HCl | Mirabegron Impurity 45 | rac-N-acetyl Mirabegron | Mirabegron Impurity 15 | Mirabegron Impurity 8 | Mirabegron Impurity 10 |