Zanubrutinib Pharmaceutical Reference Standards
ZANUBRUTINIB IMPURITIES, IMPURITIES
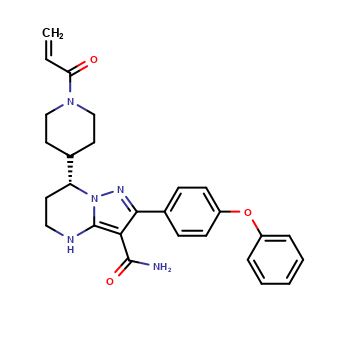
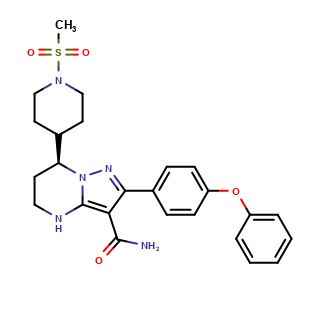
(R)-Zanubrutinib
| CAT No. |
: CS-O-47725 |
| Mol F. |
: C27H29N5O3 |
| Mol W. |
:
471.55 g/mol
|
| Cas No. |
: 1691249-44-1 |
| Stock |
: Enquire |
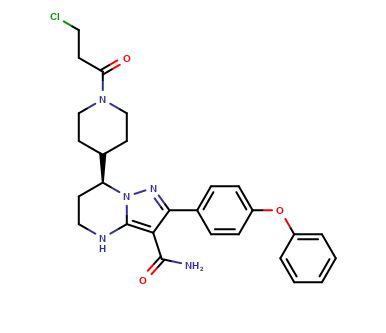
| CAT No. |
: CS-EO-03112 |
| Mol F. |
: C27H30ClN5O3 |
| Mol W. |
:
508.01 g/mol
|
| Cas No. |
: 1633351-79-7 |
| Stock |
: Enquire |
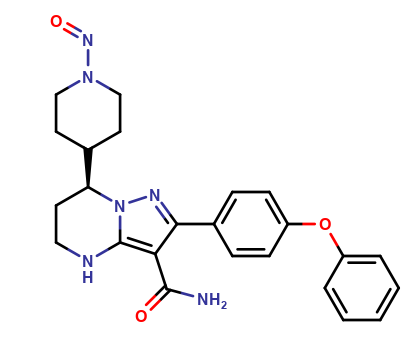
| CAT No. |
: CS-O-52284 |
| Mol F. |
: C24H26N6O3 |
| Mol W. |
:
446.51 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
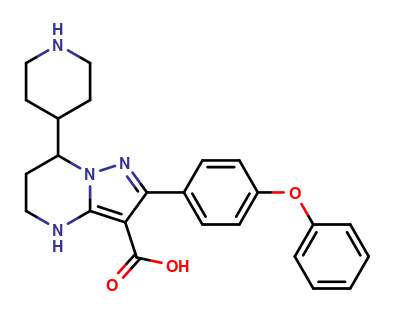
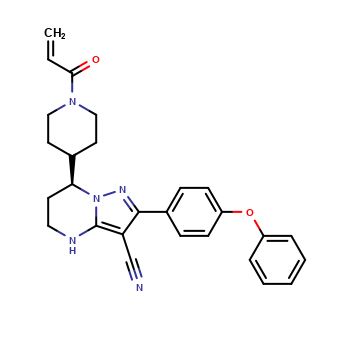
| CAT No. |
: CS-O-49682 |
| Mol F. |
: C24H26N4O3 |
| Mol W. |
:
418.49 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
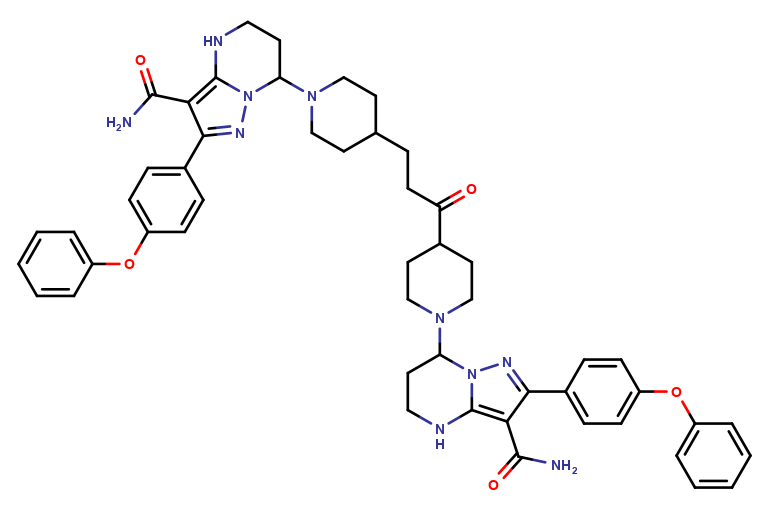
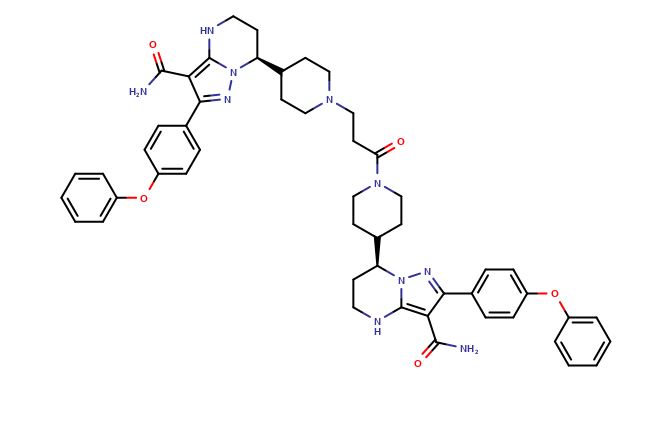
| CAT No. |
: CS-O-51889 |
| Mol F. |
: C51H56N10O5 |
| Mol W. |
:
889.05 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
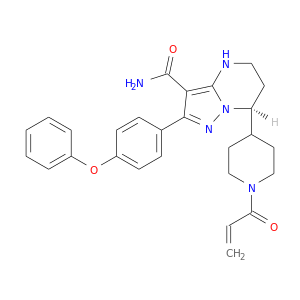
| CAT No. |
: CS-O-49683 |
| Mol F. |
: C29H29N5O3 |
| Mol W. |
:
495.57 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
ZANUBRUTINIB API STANDARDS, API STANDARDS
Zanubrutinib
| CAT No. |
: CS-ZB-15280 |
| Mol F. |
: C27H29N5O3 |
| Mol W. |
:
471.55 g/mol
|
| Cas No. |
: 1691249-45-2 |
| Stock |
: Enquire |
| CAT No. |
: CS-EO-03113 |
| Mol F. |
: C27H27N5O2 |
| Mol W. |
:
453.54 g/mol
|
| Cas No. |
: 2432022-47-2 |
| Stock |
: Enquire |
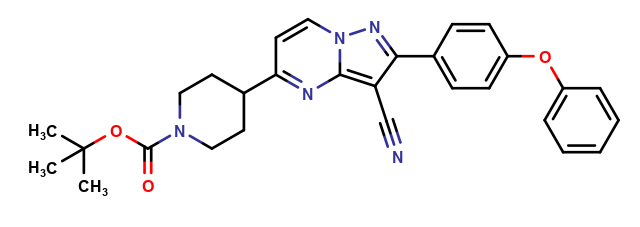
| CAT No. |
: CS-O-47726 |
| Mol F. |
: C51H56N10O5 |
| Mol W. |
:
889.05 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
| CAT No. |
: CS-O-47727 |
| Mol F. |
: C25H29N5O4S |
| Mol W. |
:
495.59 g/mol
|
| Cas No. |
: Not Available |
| Stock |
: Enquire |
ZANUBRUTINIB STABLE ISOTOPES, STABLE ISOTOPES
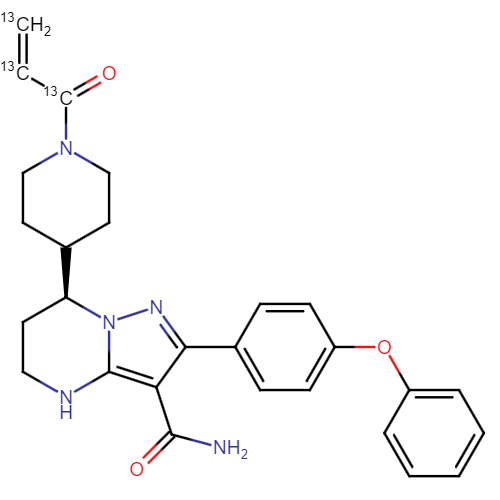
Zanubrutinib-13C3
| CAT No. |
: CS-T-95963 |
| Mol F. |
: C2413C3H29N5O3 |
| Mol W. |
:
474.54 g/mol
|
| Cas No. |
: 1691249-45-2 (Unlabelled) |
| Stock |
: Enquire |
ZANUBRUTINIB STABLE ISOTOPES, STABLE ISOTOPES
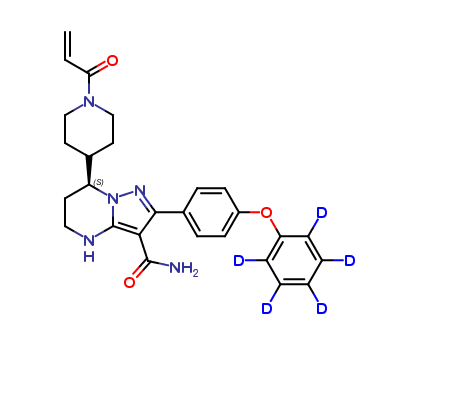
Zanubrutinib-D5
| CAT No. |
: CS-P-08541 |
| Mol F. |
: C27H24D5N5O3 |
| Mol W. |
:
476.59 g/mol
|
| Cas No. |
: 1691249-45-2 (Unlabeled) |
| Stock |
: Enquire |