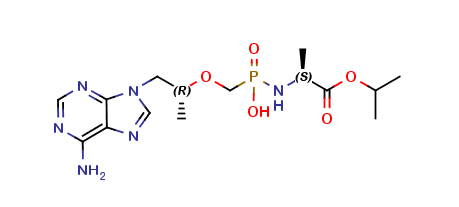
Tenofovir alafenamide Impurity 1
| Product Name | Tenofovir alafenamide Impurity 1 |
|---|---|
| Alternate Names | Tenofovir Impurities, Impurities of Tenofovir |
| CAT No. | CS-O-15852 |
| CAS No. | 851456-00-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | Not Available |
| Mol. For. | Not Available |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Tenofovir |
| Therapeutic | Antiretroviral / Anti-HIV |
| Smileys | C[C@@H](OC[P](N[C@@H](C)C(OC(C)C)=O)(O)=O)CN1C2=NC=NC(N)=C2N=C1 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Tenofovir alafenamide Impurity 1 is a chemical compound that is used in the pharmaceutical industry as a reference standard to determine the purity of Tenofovir alafenamide (TAF), a medication used to treat Human Immunodeficiency Virus (HIV) and Hepatitis B Virus (HBV) infections. TAF is a prodrug of Tenofovir, which is an antiviral medication. TAF is metabolized in the body to Tenofovir, which then inhibits the viral reverse transcriptase enzyme, thereby preventing the replication of the virus.
Tenofovir alafenamide Impurity 1 is a synthetic compound that is chemically known as 3-((R)-1-(6-amino-9H-purin-9-yl)propan-2-yloxy)-5-methylbenzonitrile. It is a white or off-white crystalline powder that has a molecular weight of 323.32 grams per mole. Tenofovir alafenamide Impurity 1 is soluble in organic solvents such as methanol, ethanol, and dimethyl sulfoxide.
The usage of Tenofovir alafenamide Impurity 1 is primarily in quality control and analytical testing of TAF. It is used as a standard reference material for high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) analysis. The purity of TAF is determined by comparing the retention time and peak area of Tenofovir alafenamide Impurity 1 with that of the TAF sample.
In conclusion, Tenofovir alafenamide Impurity 1 is an important reference standard for the quality control of TAF, which is a widely used medication for the treatment of HIV and HBV infections. Its chemical properties and usage in analytical testing make it a valuable tool for pharmaceutical companies and research institutions.
Get an Instant Quote
Related Compounds
Tenofovir Alafenamide Enantiomer | MOC-POC Tenofovir Fumarate | Tenofovir DP-IV | TAF Propyl ester Impurity | Tenofovir Impurity 35 | Mono-POC Methyl Tenofovir Fumarate (Mixture of Diastereomers) | Tenofovir Alafenamide N-hydroxy methyl impurity | Tenofovir Disoproxil T8 Impurity | Tenofovir disoproxil impurity L fumarate | Tenofovir Alafenamide Dimer impurity | Tenofovir Alanine ester impurity | Mono-POC Ethyl Tenofovir | Tenofovir Mixed dimer | Tenofovir impurity C fumarate salt | Tenofovir EP Impurity C Succinate | Tenofovir alafenamide Impurity 3 | Tenofovir DP-V | Tenofovir disoproxil impurity L | Diethylaminocarboxymethyl POC Tenofovir (Mixture of Diastereomers) | Tenofovir Disoproxil Dimer | Tenofovir alafenamide (RRS) diasteroisomer | Tenofovir alafenamide lactose Millard Reaction product-2 | tenofovir related impurity | Tenofovir alafenamide lactose Millard Reaction product-3 | Tenofovir Disoproxil Isopropoxycarbonyl | Tenofovir Related Compound 5 | Tenofovir Monomethyl Ester | Tenofovir Bis ((R)-9-(2-Hydroxypropyl)adenine) dimer Acetate salt | Diisopropyl Tenofovir Fumarate | Tenofovir Alfenamide Amadori Rearrangement product | Mono ester impurity (Tenofovir) | Tenofovir Disoproxil T11 Impurity | Tenofovir isomer 6&1 | Tenofovir Impurity 89 | Mono-POC Tenofovir 6-Isopropyl Carbamate | Isopropyl Tenofovir | Tenofovir Disoproxil T12 Impurity | Tenofovir Mono POC Dimer Diethyl amine salt | MOC-POC Tenofovir | Tri-POC Tenofovir Dimer | Tenofovir Disoproxil T7 Impurity | TAF methyl ester | Tenofovir Alafenamide (RSR) Diasteroisomer | Tenofovir alafenamide monofumarate | Tenofovir Mono-POC methyl ester | Tenofovir Impurity 59-D6 | Tenofovir Disoproxil T5 Impurity | Desmethyl Tenofovir disoproxil | Tenofovir disoproxil phosphate | Tenofovir Alafenamide fumarate | TAF SRS diastereomer | Tenofovir Related Compound 14 | Tenofovir alafenamide Impurity 2 | Tenofovir Disoproxil Fumarate Impurity | Mono poc-isopropyl-Tenofovir fumarate salt | Tenofovir Disoproxil (S)-Isomer fumarate salt | Tenofovir Impurity 59 | Tenofovir Alafenamide Glycosamine product | Mono-POC Isopropyl Tenofovir | Tenofovir diphosphate | nPOC-POC Tenofovir | Ethyl Tenofovir Impurity | Tenofovir Isopropyl Carbamate | Tenofovir Impurity 65 | Tenofovir Impurity 36 (RSR) 2,3-dihydroxysuccinate salt | Diethylaminocarboxymethyl POC Tenofovir Fumarate | nPOC-POC Tenofovir Fumarate | Tenofovir Alafenamide Dimer Impurity II | Tenofovir Impurity 39 (RRR) 2,3-dihydroxysuccinate salt | Tenofovir Diphenyl HemiFumarate salt |