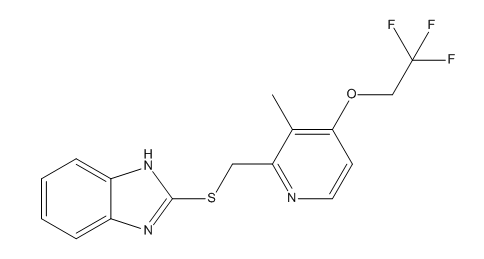
Lansoprazole Sulfide Impurity
| Product Name | Lansoprazole Sulfide Impurity |
|---|---|
| Alternate Names | Lansoprazole Impurities, Impurities of Lansoprazole |
| CAT No. | CS-O-31884 |
| CAS No. | 103577-40-8 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 353.36 g/mol |
| Mol. For. | C₁₆H₁₄F₃N₃OS |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Lansoprazole |
| Purity | 95% |
| Therapeutic | Anti ulcer |
| Smileys | CC1=C(N=CC=C1OCC(F)(F)F)CSC2=NC(C=CC=C3)=C3N2 |
| Canonical Smiles | CC1=C(C=CN=C1CSC2=NC3=CC=CC=C3N2)OCC(F)(F)F |
| InchIKey | CCHLMSUZHFPSFC-UHFFFAOYSA-N |
| Inchl | InChI=1S/C16H14F3N3OS/c1-10-13(20-7-6-14(10)23-9-16(17,18)19)8-24-15-21-11-4-2-3-5-12(11)22-15/h2-7H,8-9H2,1H3,(H,21,22) |
| IUPAC | 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfanyl]-1H-benzimidazole |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Lansoprazole Sulfide Impurity is a chemical compound that is commonly used in the pharmaceutical industry. It is a byproduct of the synthesis of Lansoprazole, which is a medication used to treat gastroesophageal reflux disease (GERD), stomach ulcers, and other related conditions. The impurity is formed during the chemical reaction that produces Lansoprazole, and it is considered a minor impurity in the final product.
The chemical structure of Lansoprazole Sulfide Impurity is similar to that of Lansoprazole, but it contains a sulfur atom in place of an oxygen atom. This substitution affects the chemical properties of the molecule, making it less effective in treating GERD and other conditions. Therefore, it is important to monitor and control the level of this impurity in the final product to ensure its safety and efficacy.
To detect and quantify Lansoprazole Sulfide Impurity, various analytical techniques such as high-performance liquid chromatography (HPLC) and gas chromatography (GC) are used. These methods allow for accurate measurement of the impurity level and ensure that it meets the required specifications set by regulatory authorities.
In conclusion, Lansoprazole Sulfide Impurity is an important chemical compound in the pharmaceutical industry, and its presence in Lansoprazole must be monitored and controlled to ensure the safety and efficacy of the medication.
Get an Instant Quote
Related Compounds
N-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl Lansoprazole Sulfide | Lansoprazole Desulphur Impurity | Lansoprazole D3 | Lansoprazole Sulfide N-Oxide | N-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl Lansoprazole Sulfone | Lansoprazole Thioxo Impurity | N-Nitroso Lansoprazole sulfide | Nitroso Lansoprazole | Lansoprazole Related Compound B | Lansoprazole Pyridinone impurity | Lansoprazole Related Compound 4 | Lansoprazole Cyclized Impurity | Lansoprazole Related Compound 6 | Lansoprazole Impurity 33 | N-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl Lansoprazole | Lansoprazole Base hydrolysis | Lansoprazole impurity(M+467) | Destrifluoro ethoxy Lansoprazole | Lansoprazole Related Compound 5 | Lansoprazole Impurity 1 |