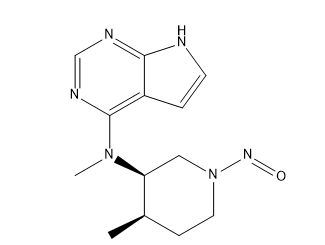
N-Nitroso Tofacitinib Amine Impurity
Also known as: Tofacitinib Nitrosamine Impurities or nitrosamine impurities of Tofacitinib| Product Name | N-Nitroso Tofacitinib Amine Impurity |
|---|---|
| Alternate Names | Tofacitinib Impurities, Impurities of Tofacitinib |
| CAT No. | CS-O-40197 |
| CAS No. | Not Available |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 274.32 g/mol |
| Mol. For. | C₁₃H₁₈N₆O |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Tofacitinib |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
N-Nitroso Tofacitinib Amine Impurity is a chemical compound that is commonly found as an impurity in Tofacitinib, which is an FDA-approved medication for the treatment of rheumatoid arthritis. N-Nitroso Tofacitinib Amine Impurity is a potent carcinogen and is known to cause various types of cancers, including bladder cancer, pancreatic cancer, and stomach cancer. Therefore, it is essential to monitor the levels of this impurity in Tofacitinib formulations to ensure that they are within the acceptable limits.
The usage of N-Nitroso Tofacitinib Amine Impurity is strictly prohibited as it can cause severe harm to human health. Ingestion or exposure to this compound can lead to various health complications, including DNA damage, oxidative stress, and mutations. The chemical structure of N-Nitroso Tofacitinib Amine Impurity consists of a nitroso group (-NO) and an amine group (-NH2), which make it highly reactive and unstable.
To minimize the risk of exposure to N-Nitroso Tofacitinib Amine Impurity, pharmaceutical companies are required to perform routine tests and analysis to ensure that the levels of this impurity are below the acceptable limits. The acceptable limit of N-Nitroso Tofacitinib Amine Impurity in Tofacitinib formulations is set by regulatory authorities such as the FDA and the European Medicines Agency (EMA) to ensure patient safety.
Get an Instant Quote
Related Compounds
Tofacitinib formyl impurity | N,4-dimethyl-1-nitrosopiperidin-3-amine | Tofacitinib impurity P | Tofacitinib Hydroxy Impurity | Tofacitinib Nitroso Impurity 5 | Tofacitinib Dihydro Impurity | N-(1-benzyl-4-methylpiperidin-3-yl)-N-methylnitrous amide | Tofacitinib impurity T | Tofacitinib Impurity 6 | TOFACITINIB IMPURITY TOF-IV | Tofacitinib butyl ester impurity | Tofacitinib impurity Z | Tofacitinib Impurity 16 | Tofacitinib Impurity 29 | Tofacitinib Nitroso Impurity 4 | Tofacitinib impurity C | Tofacitinib Related Compound 29 | Tofacitinib- Keto Impurity | Tofacitinib related compound 6 | Tofacitinib Nitroso Impurity 2 | Tofacitinib Diastereomer | Tofacitinib Nitroso Impurity | N-methyl-N-(4-methylpiperidin-3-yl)nitrous amide | Tofacitinib N-Nitroso benzyl intermediate | Tofacitinib N-oxide | Tofacitinib Nitroso Impurity 1 | Tofacitinib Impurity 36 | N-methyl-N-(4-methylpyridin-3-yl)nitrous amide | Tofacitinib Diastereomer-1 and 2 | Tofacitinib Impurity 57 | Tofacitinib impurity I | Tofacitinib Dihydro Citricacid salt impurity | Tofacitinib N- acid Impurity | Tofacitinib Related Compound 26 HCl | Tofacitinib S,S Isomer | Tofacitinib N-hydroxy impurity | Tofacitinib Impurity (N-Des-(2-Cyanide-acetyl)-(3S,4R)) | Tofacitinib impurity N | Tofacitinib impurity G | N-methyl-N-(4-methyl-1-nitrosopiperidin-3-yl)nitrous amide | Tofacitinib Nitroso Impurity 3 | Tofacitinib impurity J | Tofacitinib impurity V | Tofacitinib impurity K |