Semiconductors
How Deuterium Gas is Used in Semiconductors?
Deuterium gas (2H2; D2) is used in the manufacturing of silicon semiconductors and microchips found commonly in circuit boards through the process of a deuterium-protium exchange. Deuterium annealing replaces the protium atoms with deuterium, preventing deterioration of the chip circuitry from chemical erosion and the Hot Carrier Effect. This process significantly extends and improves the life cycle of semiconductors and microchips, and also allows them to be made smaller and have high circuit densities (high-density chips).
What is the “Hot Carrier Effect”?
The Hot Carrier Effect refers to the degradation or instability caused by Hot Carrier Injection, which ultimately lowers the lifespan of a chip. This Hot Carrier Injection issue occurs when an electron gains enough kinetic energy to overcome an electric potential barrier and break through an interface state. After this occurs, they typically then hit a Si-H bond, and in the process, break it. As these bonds are being broken inside the transistors in the semiconductor, the chip slowly starts to degrade until it is no longer functional. A close up picture of a circuit showcasing D2O and its place within the semiconductor industryThere is no exact point at which the Hot Carrier Effect begins to occur to semiconductors, but rather it is essentially random.
The degradation effects associated with the Hot Carrier Effect essentially set a limit to the lifetime of a transistor and, therefore, must be controlled as well as possibly maximize the lifespan of a device. A solution to help relieve some of the degradation effects is to utilize deuterium through an annealing process.
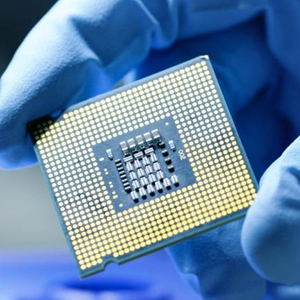
What Are Semiconductors?
Semiconductors, in the simplest terms, are materials whose conductivity is less than that of a conductor (i.e. copper), but greater than that of an insulator (i.e. glass).
The most popular material used within integrated circuits such as semiconductors is Silicon, and less commonly Gallium, Arsenide, Silicon Carbide, and Germanium. However, the commonly used Silicon in its pure form does not have the capacity to perform well electrically, so it must first undergo a “Doping” process.
What is Semiconductor Doping?
The word “Doping” refers to the process of purposefully introducing other elements (impurities) into a pure crystal to modulate the electric properties of the material. Depending on the purpose and end-use of the semiconductor, the level of doping and the chemical done with varies. However, typically doping is performed with Boron or Phosphorus as they are elements with one less and one surplus valence electron, as compared to Silicon’s four valence electrons. Depending on the doping process, there are two possible outcomes from Silicon doping: N-Type, and P-Type; each of which give the semiconductors specific properties.
Need more information about our value added D2O products?
Connect with us and share a brief about your requirement, we will get in touch with you with a detailed information.
Brief History of Semiconductors
The semiconductor effect was first documented in 1833 when British physicist Michael Faraday noted that the electrical resistance of Silver sulphide declines as temperatures fall. Later, in 1874, the next big break through came when British physicist Arthur Schuster observed the process of rectification in his circuit made of copper wires. It was only in 1929, however, that German physicist Walter Schottky was able to confirm the theories that there was “semiconductor effect” during his metal-semiconductor experiment.
In 1876, William Grylls Adams and Richard Evans Day discovered that by lighting up a junction between platinum and selenium, the direction of the electric current could change. Through this discovery, it led to the creation of the world’s first solar cell in 1883 by Charles Fritts, who unknowingly at the time was applying the “semiconductor effect”.
Into the 21st century, American physicists John Bardeen and Walter Brattain developed the first semiconductor transistor in 1947. The transistor today is a staple in essentially all modern technologies. It is the component which allows practically all technology to function, with each computer produced today having billions inside.
The Benefits of Using Deuterium in Semiconductors
There are a variety of benefits that come from using Deuterium in semiconductors rather than Protium (the common isotope of Hydrogen). Firstly, a Si-D bond has a much shorter vibrational relaxation time than the traditional Si-H bond. What this means is that the compound approaches a vibrational equilibrium point much more quickly. The reason for this has been attributed to a quantum coupling with an Si-Si bond deep in the silicon crystal. Overall, this leads to a dramatically longer lifespan of D-doped devices over H-doped.
Through studies it has been identified that there is an unusual amount of synchronicity between Deuterium and Silicon. What this means is that the two elements bond very well together, very quickly, and very strongly; all of which are good for the manufacturing and use of semiconductors chips and microchips
Deuterium has been found to reduce the severity of Hot Carrier Effects that act upon semiconductors, while reducing stress induced leaked currents. Both the Hot Carrier Effect and stress induced leaked currents drastically control the lifespan of a semiconductor and are typically leading causes of malfunction within a chip
To order deuterium-based products for electronics applications please contact our team at d2o@clearsynth.com