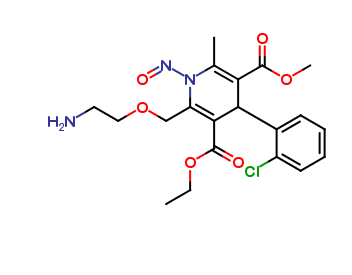
Amlodipine Nitroso Impurity
Also known as: Amlodipine Nitrosamine Impurities or nitrosamine impurities of Amlodipine| Product Name | Amlodipine Nitroso Impurity |
|---|---|
| Alternate Names | Amlodipine Impurities, Impurities of Amlodipine |
| CAT No. | CS-EO-01203 |
| CAS No. | Not Available |
| Category | Impurities |
| Stock | Enquire |
| Mol. Wt. | 437.9 g/mol |
| Mol. For. | C20H24ClN3O6 |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Amlodipine |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Amlodipine is a commonly prescribed medication for the treatment of hypertension and angina. It is a calcium channel blocker that works by relaxing the blood vessels and improving blood flow. However, during the manufacturing process of amlodipine, an impurity known as amlodipine nitroso impurity (ANI) can be formed.
ANI is a potentially harmful impurity that can negatively affect the efficacy and safety of amlodipine. It is classified as a nitroso compound, which means it contains a nitroso group (-NO) attached to a carbon atom. Nitroso compounds have been found to be carcinogenic and mutagenic in animal studies.
Therefore, it is essential to monitor and control the levels of ANI in amlodipine products to ensure their safety and efficacy. The acceptable limit of ANI in amlodipine products is set at 0.15% by most regulatory agencies.
Analytical methods such as high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) are commonly used to detect and quantify ANI in amlodipine products.
In conclusion, ANI is a potentially harmful impurity that can form during the manufacturing process of amlodipine. Its levels must be monitored and controlled to ensure the safety and efficacy of amlodipine products.
Get an Instant Quote
Related Compounds
Amlodipine Related Compound A | Amlodipine Impurity 32 | Desmethyl Amlodipine | Phthaloyl Amlodipine Dimethyl Ester | Amlodipine N-Galactose | Amlodipine EP Impurity B | Amlodipine Impurity 29 | Amlodipine Galactose Adduct | Amlodipine EP Impurity E | Amlodipine Aspartic Acid Impurity | Amlodipine Impurity 34 | Amlodipine orotate | N-Nitroso Amlodipine | R-Amlodipine Besilate | Amlodipine Impurity | Amlodipine Impurity F Maleate | Atorvastatin-Amlodipine Adduct | Amlodipine Impurity 19 | Amlodipine EP Impurity G | Amlodipine Impurity 38 | Amlodipine EP Impurity F | Amlodipine Impurity 25 | Amlodipin EP Impurity D | Amlodipine aminoethyl | Amlodipine EP Impurity E Maleate | Amlodipine Impurity 61 | Amlodipine EP Impurity D Oxalate Salt | R-Amlodipine hemipentahydrate | Amlodipine For Peak Identification | Amlodipine Lactose adduct | Deschloro Amlodipine | Amlodipine morpholine impurity | Amlodipine Azido Impurity | Amlodipine Mannitol Adduct | Amlodipine Besylate Impurity D Besylate salt | Amlodipine N-Lactoside | Dimethoxy Impurity Deschloro Amlodipine | Amlodipine Impurity 8 | Amlodipine Mannitol Adduct HCl Salt | Amlodipine EP Impurity D HCl | Amlodipine Methyl Ester | Di-acid Amlodipine | Dehydro Amlodipine N-Oxide | Amlodipine Mannitol Adduct Acetate Salt | Atorvastatin Amlodipine Dimer | N-Fomyl Amlodipine | N-Nitroso Amlodipine EP Impurity F | Hydroxyethyl phthalyl amlodipine | Amlodipine EP Impurity A | Amlodipine EP Impurity H | Amlodipine Impurity 9 | Amlodipine EP Impurity C |