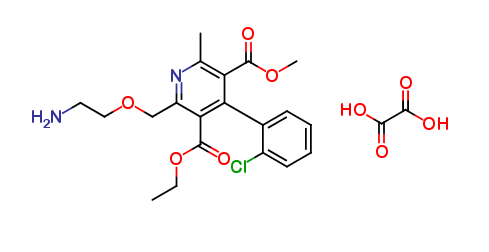
Amlodipine EP Impurity D Oxalate Salt
| Product Name | Amlodipine EP Impurity D Oxalate Salt |
|---|---|
| Alternate Names | Amlodipine Impurities, Impurities of Amlodipine |
| CAT No. | CS-O-07176 |
| CAS No. | 1216406-90-4 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 496.89 g/mol |
| Mol. For. | C₂₂H₂₅ClN₂O₉ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Amlodipine |
| Purity | 95% |
| Therapeutic | Anti-Hypertensives |
| Smileys | CCOC(=O)C1=C(C(=C(N=C1COCCN)C)C(=O)OC)C2=CC=CC=C2Cl.C(=O)(C(=O)O)O |
| Canonical Smiles | CCOC(=O)C1=C(C(=C(N=C1COCCN)C)C(=O)OC)C2=CC=CC=C2Cl.C(=O)(C(=O)O)O |
| InchIKey | VAHKAMHZXZAAGV-UHFFFAOYSA-N |
| Inchl | InChI=1S/C20H23ClN2O5.C2H2O4/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21;3-1(4)2(5)6/h5-8H,4,9-11,22H2,1-3H3;(H,3,4)(H,5,6) |
| IUPAC | 3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methylpyridine-3,5-dicarboxylate;oxalic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Amlodipine EP Impurity D Oxalate Salt is a chemical compound that is commonly used in the pharmaceutical industry. It is a derivative of the drug Amlodipine, which is a calcium channel blocker that is used to treat hypertension and angina. This impurity is produced during the synthesis of Amlodipine and is considered to be an unwanted byproduct that needs to be removed from the final product.
The chemical formula of Amlodipine EP Impurity D Oxalate Salt is C20H25ClN2O8, and it has a molecular weight of 456.88 g/mol. This compound is a white to off-white crystalline powder that is soluble in water and ethanol. It is also stable under normal conditions of storage and handling.
Although Amlodipine EP Impurity D Oxalate Salt is not used as a drug itself, it is important to monitor its presence in Amlodipine samples. This is because the impurity can affect the purity and potency of the final drug product. As such, regulatory agencies such as the US Food and Drug Administration (FDA) have set limits on the amount of impurities that are allowed in drug products.
In summary, Amlodipine EP Impurity D Oxalate Salt is an unwanted impurity that is produced during the synthesis of Amlodipine. It needs to be monitored and controlled to ensure the purity and potency of the final drug product.
Get an Instant Quote
Related Compounds
Amlodipine Impurity 9 | Amlodipine For Peak Identification | Amlodipine Impurity F Maleate | Amlodipine Mannitol Adduct HCl Salt | R-Amlodipine hemipentahydrate | Amlodipine Impurity 19 | Di-acid Amlodipine | Amlodipine N-Lactoside | Phthaloyl Amlodipine Dimethyl Ester | Amlodipine Methyl Ester | Amlodipine Galactose Adduct | Amlodipine Mannitol Adduct | Amlodipine Aspartic Acid Impurity | Atorvastatin Amlodipine Dimer | Dehydro Amlodipine N-Oxide | Amlodipine Impurity 34 | Amlodipine Impurity 38 | Amlodipine Impurity | Amlodipine Impurity 32 | Amlodipine EP Impurity A | Amlodipine N-Galactose | Amlodipine Related Compound A | Amlodipin EP Impurity D | Deschloro Amlodipine | Hydroxyethyl phthalyl amlodipine | Amlodipine EP Impurity H | Amlodipine aminoethyl | Amlodipine EP Impurity F | Amlodipine Azido Impurity | Amlodipine Besylate Impurity D Besylate salt | Amlodipine EP Impurity C | Amlodipine Impurity 29 | Amlodipine Mannitol Adduct Acetate Salt | Dimethoxy Impurity Deschloro Amlodipine | Amlodipine EP Impurity E | Amlodipine EP Impurity E Maleate | Atorvastatin-Amlodipine Adduct | Amlodipine orotate | Desmethyl Amlodipine | N-Nitroso Amlodipine | Amlodipine EP Impurity B | Amlodipine Nitroso Impurity | N-Fomyl Amlodipine | R-Amlodipine Besilate | Amlodipine Impurity 61 | Amlodipine morpholine impurity | Amlodipine EP Impurity D HCl | Amlodipine Impurity 25 | Amlodipine EP Impurity G | Amlodipine Impurity 8 | Amlodipine Lactose adduct | N-Nitroso Amlodipine EP Impurity F |