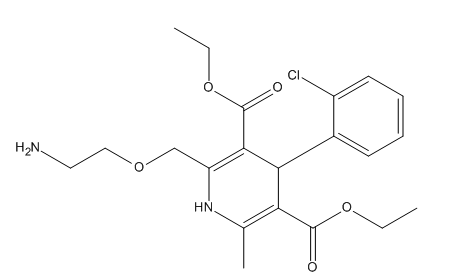
Amlodipine EP Impurity E
| Product Name | Amlodipine EP Impurity E |
|---|---|
| Alternate Names | Amlodipine Impurities, Impurities of Amlodipine |
| CAT No. | CS-O-07177 |
| CAS No. | 140171-65-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 422.90 g/mol |
| Mol. For. | C₂₁H₂₇ClN₂O₅ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Amlodipine |
| Purity | 95% |
| Therapeutic | Anti-Hypertensives |
| Smileys | O=C(C(C(C(C=CC=C1)=C1Cl)C(C(OCC)=O)=C(C)N2)=C2COCCN)OCC |
| Canonical Smiles | CCOC(=O)C1=C(NC(=C(C1C2=CC=CC=C2Cl)C(=O)OCC)COCCN)C |
| InchIKey | BGGLOZPVAWMSEB-UHFFFAOYSA-N |
| Inchl | InChI=1S/C21H27ClN2O5/c1-4-28-20(25)17-13(3)24-16(12-27-11-10-23)19(21(26)29-5-2)18(17)14-8-6-7-9-15(14)22/h6-9,18,24H,4-5,10-12,23H2,1-3H3 |
| IUPAC | diethyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Amlodipine EP Impurity E, also known as 2-(2-Benzofuranyl)ethanol, is a chemical impurity that may be present in Amlodipine besylate, a widely prescribed medication used to treat high blood pressure and angina.
The usage of Amlodipine EP Impurity E is mainly for analytical purposes, as it can be used as a reference standard for the identification and quantification of impurities in Amlodipine besylate. It is also used in the development and validation of analytical methods for the quality control of Amlodipine besylate in pharmaceutical manufacturing.
The chemical information of Amlodipine EP Impurity E includes its molecular formula, C10H10O2, and its molecular weight, 162.19 g/mol. It is a white to off-white crystalline powder that is sparingly soluble in water and soluble in organic solvents such as methanol and ethanol.
In terms of safety, Amlodipine EP Impurity E is not intended for human consumption and should be handled with care in a laboratory setting. It is important to follow proper handling and disposal procedures to ensure the safety of laboratory personnel and the environment.
In conclusion, Amlodipine EP Impurity E is a chemical impurity that is mainly used for analytical purposes in the pharmaceutical industry. Its chemical information and properties should be understood and handled with care in a laboratory setting.
Get an Instant Quote
Related Compounds
Amlodipin EP Impurity D | Amlodipine Impurity 32 | Amlodipine Impurity 25 | Amlodipine Impurity 34 | R-Amlodipine Besilate | Amlodipine Impurity 19 | Amlodipine For Peak Identification | Dehydro Amlodipine N-Oxide | Amlodipine Galactose Adduct | Atorvastatin-Amlodipine Adduct | Amlodipine EP Impurity H | Amlodipine Impurity 8 | Dimethoxy Impurity Deschloro Amlodipine | Amlodipine Aspartic Acid Impurity | Amlodipine EP Impurity A | Amlodipine Mannitol Adduct Acetate Salt | Amlodipine N-Galactose | Amlodipine EP Impurity B | Amlodipine Besylate Impurity D Besylate salt | N-Nitroso Amlodipine | Amlodipine Impurity 29 | Amlodipine EP Impurity C | Amlodipine orotate | Desmethyl Amlodipine | Amlodipine Nitroso Impurity | Amlodipine EP Impurity D HCl | N-Fomyl Amlodipine | Atorvastatin Amlodipine Dimer | Amlodipine Impurity F Maleate | N-Nitroso Amlodipine EP Impurity F | Amlodipine aminoethyl | Hydroxyethyl phthalyl amlodipine | Amlodipine morpholine impurity | Amlodipine EP Impurity D Oxalate Salt | Amlodipine Mannitol Adduct | Amlodipine EP Impurity F | Amlodipine Impurity 61 | Amlodipine EP Impurity E Maleate | R-Amlodipine hemipentahydrate | Amlodipine Lactose adduct | Amlodipine Related Compound A | Deschloro Amlodipine | Amlodipine EP Impurity G | Amlodipine Azido Impurity | Phthaloyl Amlodipine Dimethyl Ester | Di-acid Amlodipine | Amlodipine Impurity 38 | Amlodipine Impurity 9 | Amlodipine Mannitol Adduct HCl Salt | Amlodipine Methyl Ester | Amlodipine N-Lactoside | Amlodipine Impurity |